Why Atoms Ever Get Together
In answering the question of why the different elements associate in the manner in which they obviously do, the first, and most important factor, is, of course, the incessant progression of motion. The second most important factor is the net effective speed displacement at the individual reference point. The third factor involves the relations among the specific modes of displacement represented at each reference point involved in an association.
The first positive 2D1dR displacement creating the rotational base completely offset the possibility of direct representation of linear outward progression for individual Notational Reference Points. As a result of random orientation of atomic coordinate systems, subsequent rotationally represented positive displacements cause each such compound motion structure to have the scalar effect of progressing inward toward all spatial locations in the natural reference system. The ambiguity of direction for the outward progression of primary motion away from all spatial locations in the fixed spatial reference system makes it seem that all atoms and sub-atoms of matter are moving randomly outward. The consequences of compounding rotationally represented displacements, both one and two dimensional, positively and negatively displaced, along with additional 1D1dL and 1D2dL displacements cause many and varied phenomena.
Every compound motion structure that is identified as an atom or sub-atom of matter is moving inward toward all natural locations, some of which are occupied by other atoms or sub-atoms, most of which are not. We observe the movement of large aggregates of atoms of matter as collectively moving inward toward other aggregates in such a manner that their net inward progression has been identified as gravitation. The motion causing gravitation is inherently present for all atoms of matter. It is the “all directions” characteristic of rotational representation of displacement motion required in the complexation sequence for displacements represented at individual reference points for all atoms and sub-atoms of matter that is the basis for determining that it is this characteristic of the complex motion of atoms that is the cause of gravitational motion. The mathematical analysis of the behavioral characteristics of this type of motion representation supports this conclusion. The force of gravitation is simultaneously and instantaneously present with the presence of matter; it is not propagated at a finite velocity; it is either present because matter is present or it is not.
The inward force effect of rotationally represented displacements is of such a nature that the gravitational force effect cannot be screened off in any way. In a universe of motion there is no anti-gravity or artificial gravity of the “Star Trek” variety; that is strictly wishful thinking of “science fiction” engendered by the fact that the previously accepted theoretical system has no specific explanation for the cause of a gravitational effect, let alone any indication that an anti-gravity effect should not be observable.
In a universe of motion, a force is necessarily a motion or an effect of motion. We therefore define a “force” as that which will produce motion as perceived in a spatial or temporal reference system (and is thereby perceived as vectorial) if not prevented from doing so by other “forces”. “Force” is merely a special way of looking at motion, at its effect, rather than at its immediate value. It is still convenient to use the concept of force on an “as if real” basis along with the same mathematical relations among “forces” as in current usage, but with the realization that “force” is a derived concept, not something real.
Real is the actual presence of something, the actual movement of the something, or tendency of that something to move, not the derived mathematical relation for any resultant movement or tendency to change location in space. The mathematical “force” is not the reality, material things and the movements which result from the presence of compound representations of motions are the true realities of the physical universe. Lack of apparent movement may be reconciled through the procedure of showing a balance of “force” effects due to the various motions present, similar to the present procedure of balancing forces. Both the first and second conditions for equilibrium in the generalized spatial reference system are still in effect. The only thing changed is an understanding for the causes of various effects.
Since force is defined as an aspect of motion, the inward gravitational motion in three dimensional space is dependent on the geometry of such a reference system. The inward force of gravity diminishes with increasing distance becoming equal in magnitude with the force of the outward progression of the natural reference system of motion at some distance from each unit or aggregate of units of matter. The three dimensional distance away from each particular aggregate, at which the numerical value of its inward gravitational motion is equal to unity, is referred to as the gravitational limit of that aggregate.
An aggregate may be two atoms or thousands of atoms, planetary or stellar quantities; even a globular cluster or a galaxy or a cluster of galaxies constitutes an aggregate for the purpose of defining the gravitational limit for the particular aggregate. Any imbalance of the two opposing force effects near any gravitational limit accentuates the imbalance, and thus, the gravitational limit is not a position of equilibrium, but one of neutral balance.
The behavior of objects significantly outside the gravitational limits of their neighbors is such that an apparent barrier seems to exist between them which can, however, be overcome by sufficient linear movement of either object. Insufficient linear movement between apparently approaching objects results in a slowing of approach and possible deflection from a previous path. Mathematical analysis of such behavior appears as an inverse square law relation for repulsion. For individual atoms and for some small groups of atoms, the normal outer gravitational limit is between one and two units of normal progression of primary motion in the spatial aspect. For large aggregates, the gravitational limit distance is much greater.
The theoretical characteristics of gravitation in the universe of motion as derived from the postulates of the Reciprocal System of theory are in complete agreement with the empirical observations. According to this theoretical approach gravitation is simply the inward scalar effect of the motion of material units, an inherent property of the atoms and particles of matter. The same motion that makes an atom be an atom also causes it to gravitate. In a universe of motion, the only thing that can resist a change of motion is another motion. The particular motion of an atom that can cause resistance to any change of its translational motion in generalized space is the rotational representation of its net effective displacement (that which makes it be an atom). The inherent inward motion of atoms of matter, effective in all directions in space provides the inertial effect that must be offset by additional linear displacements, 1D1dL, to allow vectorial movement.
The true picture of activity in a gravitationally bound system can be understood only when the apparent movement of photons and neutrinos, as well as all forms of matter, is realized to be a result of the frame of reference by which their movement is interpreted. Photons have displacement in only one reference point dimension and have no independent motion in the two remaining dimensions. Other light-speed particles have no effective displacement in at least one dimension. This lack of effective displacement in one dimension of the individual coordinate system implies that primary motion is effective in that dimension; thus, the particle remains in the same natural location and progresses outward at the speed of light in a randomly selected direction from its point of origin in the gravitationally bound fixed reference system. Effective displacement motion is an inward motion which may have both translationally and/or rotationally distributed effects in a dimensional system. The magnitude and specific representation of the effective displacement motion stabilizes the compound motion in individual Notational Reference Point systems dispersed in a generalized three dimensional system, which gives the resulting structure its measurable properties in the generalized system. Remember, motion is a concept and our interpretation of its appearance as phenomena is a result of experience.
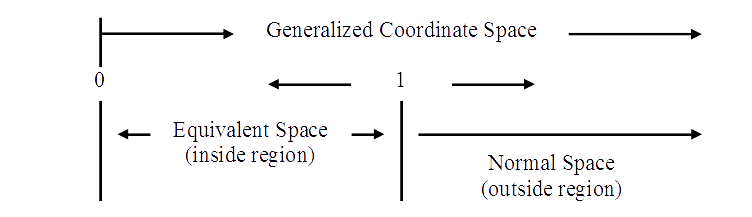
The natural progression of motion is outward from unity, 1/1. When one of the aspects of motion is confined to one unit, the progression must necessarily continue outward from that value. The outward progression from unit value may be toward larger values of separation in generalized space and time, OR as measured in a three dimensional aspect, it may be inward toward smaller values of apparent separation. If the space quantity involved in the motion is confined to one unit, the progression toward smaller values of motion is toward the mathematical zero of coordinate space.
Atoms are effectively at rest or are moving at relatively low speeds in the conventional spatial reference system because they possess inherent motions at high displacement speeds which oppose the normal outward progression of the natural reference system of motion. Because of the inward motion of each atom it becomes highly probable that some atoms will approach the same natural location and collide. The minimum amount of space is one unit, but if two atoms having momentum in generalized dimensional space approach and collide, the inability to directly represent less than one unit of space does not preclude the continuation of inward motion.
Continued apparent movement results from motion taking a random direction in three dimensional time at the unit boundary and continuing onward to establish an equilibrium position in the conventional spatial reference system that is apparently less than one unit of spatial progression. Movement to an expression of net motion as one space unit and n time units establishes the motion at 1/n net representable units of motion in the spatial aspect. Such relationship when measured in the conventional manner that uses the natural progression of time, one unit at a time, to measure distance, gives the equilibrium position an apparent distance in generalized three dimensional space related to the 1/n value of motion at equilibrium.
For a representable motion involving an effective s = 1 and t = n, the observable equivalent space is s = 1/n. The expression for speed in the normal terms of the region outside unit extension space for motion inside unit space is s/t = 1/n /n = 1/n2. Thus, the expression in the outside region for speeds in the region inside unit space is the square of the appropriate expression for the outside region; thus, inside region speed = 1/t2.13
What Holds Atoms Together
Nothing more complicated than the natural progression of scalar motion! Restating that which has been said concerning progressions and displacements for the concept of motion so as to avoid any possible ambiguity: The direction of the natural progression in coordinate space, which is always outward from unity, appears to be reversed inside unit distance in generalized space. The reversal is only an apparent one since the progression of the natural reference system is always constant in a direction outward from unit space or unit time or both unit space and unit time. Actual movement in a three dimensional reference system is due to the inward motion of the atoms of matter, and any additional linear speed displacement associated with the particles, which is always in opposition to the direction of primary motion, the motion of the natural reference system.
The “force” of gravity is defined in a three dimensional system that recognizes the mathematical zero as its reference point for numerical values. Because of the nature of a three dimensional reference system and the force of gravity always being defined as inward toward the zero of that reference system, the direction of the natural reference system inside unit space and the direction of the force of gravity are the same, toward less equivalent space.
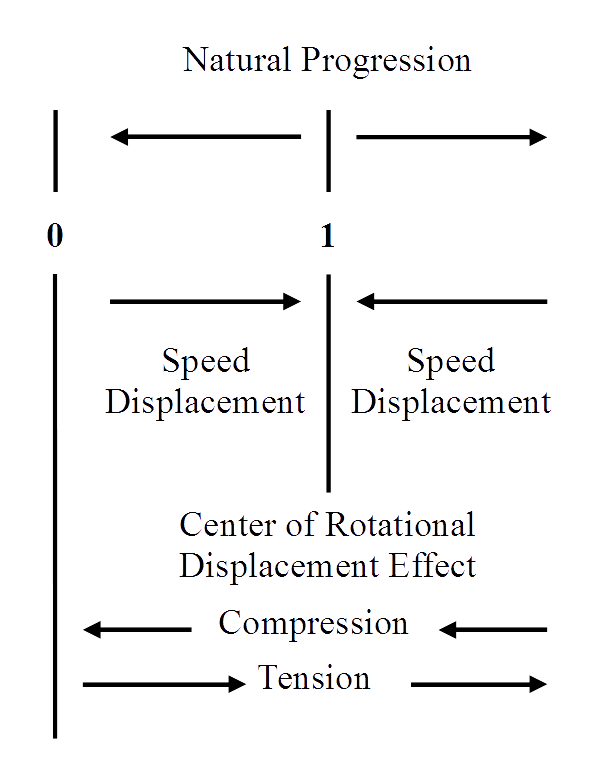
The magnitude of the force effect of the natural progression is always everywhere, in all directions, equal to unit value. At some distance from the center of rotational displacement, the force effect of the inwardly directed displacements has a magnitude of unity; equal and oppositely directed from that of the natural progression. Thinking from inside unit space, the direction of speed displacement is still toward unity, although this is now outward from zero. Since the three dimensional direction of the force effect of a speed displacement is oppositely directed inside unit space from that outside unit distance, there comes into existence an equilibrium position between any two atoms that come within unit distance of each other. They may or may not stay at that distance; that is a different question. The value of the equilibrium distance is a function of the net displacement of the individual atom; it is not caused by an interaction between atoms or an atomic size.
The scalar magnitude of the inwardly directed force due to the rotationally represented displacements varies directly with the magnitude of the specific rotational values of the atom and inversely as the square of the distance from the center of rotational action, the center of the atom. For an atom with small total displacement, the distance is very small at which the magnitude of the inwardly directed effect of the rotational displacement is equal to unity; thus, the equilibrium distance is closer to the center of the small displacement atom than for greater rotational displacement atoms. A point of equilibrium closer to the atomic center means shorter interatomic distances in generalized space.
A compressional force is inward toward zero distance of separation in generalized space, and is therefore, in the same direction inside unit space as the natural progression and thus adds to the magnitude of that force. Since the scalar magnitude of the forces due to the rotational displacements remains constant at specific distances for a given atom in a given associational relation, the force effect of compression acting together with the force effect of primary motion against the rotational forces of an atom places the point of equilibrium at shorter interatomic distance.
A force of tension in the same direction as the rotational forces, outward from zero, inward toward unit value, which adds to the rotational force or subtracts from the force effect of the natural progression of primary motion, thus requiring a greater interatomic distance for equilibrium to be established. It must be remembered that the positioning of atoms closer together than one natural unit of spatial progression is NOT a function of the other atom in the association; it is an effect of the motion that is the individual atom acting in opposition to the ever-present outward progression. The distance and direction taken by these force effects with respect to the presence of another atom is the result of the two associated atoms approaching and occupying the same natural unit of primary space and continuing their motion in dimensional time. The interatomic distance results from the interactional probability; i.e., the product of forces. The equivalent space required by each of the seemingly interacting atoms is a function of the force effect of the primary progression plus the magnitude of any externally applied compressional force or minus whatever tension force is externally applied and the effective internal pressure among the interacting atoms. Since the force due to the primary progression and a force of compression, an external pressure, act in the same direction, the equivalent of an internal pressure is present due to the primary progression acting alone against the rotational force effect. This is the cause of the so-called “chemical bonds” in molecules, crystals, glasses and true solutions.14
The zero external force equilibrium distance corresponds to the normal inter-atomic distance which is calculated from the magnitude of the effective displacements in each of the atomic dimensions for each of the associated atoms. Individual magnetic and electric displacements are actions inside unit space, and are therefore, in dimensional time. The force effect of each displacement unit is transmitted across the unit boundary in a manner dictated by the number of possible orientations for the axes of the rotational system of the atom in the time region. This is a function of the number of rotational units required for equivalency to linear units in the three dimensions required for representation and the maximum magnitude of the possible displacements in each of those dimensions.
Orientation Requirements Among Atoms for Bonding
The question concerning how many atoms of this element associate with how many atoms of that element is a function only of the effective displacements exhibited by each atom and what kind of displacement each has. The overall effect in three dimensional space is determined by the effective net displacement of each atom.
A smaller negative displacement has a more negative effect than a greater negative electric displacement because the smaller negative electric displacement in the ground state configuration is farther from the midpoint where positive and negative electric displacements have equal probability. A single negative electric displacement is far more probable than the necessary corresponding positive displacement on the preceding magnetic base. Within any one long row of the periodic charts, negativities increase in the same order as the increase in net equivalent positive electric displacement; atomic number.
In a vertical column where the electric displacements are equal, the element with the smaller magnetic displacement is the more negative because the effect of a greater magnetic displacement is to distribute the negative electric rotation over a larger total positive displacement. The magnitude of the variation in negativity due to magnetic displacement differences with the same electric displacement is less than the effect of differences in electric displacements on the same magnetic displacement. In other words, there is a greater negativity difference between adjacent elements in a given magnetic row than for adjacent elements in a given electric row.
Apparent atomic interactions place the atoms within unit primary distance in space, which we have called equivalent space. In equivalent space the dimensions of time are independent separate dimensions and thus require the displacements, in both the magnetic and the electric dimensions, to act independently.
The effective rotational displacement in the dimension of interaction determines the combining power or valence of each atom of an element. The negative valence of any atom of an element is the number of units of negative electric displacement which that atom possesses or is exhibiting in that orientation. The positive valence of an atom in a particular orientation is the number of units of negative displacement which it is able to neutralize with that orientation. The orientation determines the valence which then may result in establishment of an equilibrium distance and, thus, an association geometry in space.
Each specific structure has its maximum probability for stability in accord with the total amount of displacement motion being exhibited in the nearby environment; i.e., within one unit of space as defined by the primary progression of motion in three dimensional space.
The natural progression cannot be observed independently. Any observed phenomena must involve displacement motion whether as some form of radiation transferring rapidly by emission and absorption among material structures, actually remaining as an integral part of a specific material structure, or by the phenomena of positional relationships of material structures.
In any true chemical compound, one component must have a negative displacement, or the equivalent of a negative displacement, of four or less. A component in a compound may be a single atom or a small group of atoms which, because of their individual displacements, can collectively exhibit an appropriate negative or positive displacement. The most probable orientation in an electric or magnetic dimension, considered independently, is that orientation which causes a minimum effective displacement, zero or a value equivalent to zero, in the dimension of interaction between the atoms of the resulting structure. It is the presence of negative electric displacement available in certain elements that makes it possible to place quantitative limits on the establishment of equilibria with other atoms, and thereby, create molecules, whether with atoms of the same element or among atoms of different elements.
An equilibrium distance can be established between atoms or groups of atoms if both of the interacting atoms or groups are specifically oriented along a line of interaction. The line of interaction is in equivalent space not dimensional space, even though a line can be defined by the subsequent spatial geometry established. The equilibrium is established only when the magnitude of the resulting relative motion in the orientation dimension is unity, zero with respect to the natural datum.15 The relative motion within unit space along the lines of interaction must be reduced to zero in the natural system by the negative displacement of one atom being counterbalanced or offset by an equal positive displacement of some other atom or at least reduced to an equivalent of zero displacement in the temporal dimensions involved.
Another way of saying this is that, for formation of a molecule or radical structure, equilibrium can be established only when the net total of positive and negative displacements is zero, or the equivalent of zero, in the line or lines of interaction. This may be along the electric axis of each of the interacting atoms or an electric axis of one atom oriented with the magnetic axis of another atom. We are reserving the terms “bond” and “bonding” to that aspect of the atomic associations in which the force aspect of the progression is actually involved.
Formulas and Valence
In this approach there is no need to memorize valences, although later on it may seem as though you have learned them by memorizing. It will have been done by sufficient use of a specific technique and the normal function of the mind to remember those things it does repeatedly. Valences are what they are, and thereby, remain essentially the same as those which you may already know. The reasons for being those values will obviously change since we are no longer dealing with the idea of a nuclear atom or the concomitant ideas of electron sharing or transfer.
Valence, the chemical combining power of an atom, is determined by either the electric displacement or one of the magnetic displacements of each individual atom. Interatomic distance is affected by both the electric and magnetic displacements because distance is an effect measured from outside unit distance for an inside unit value phenomena. Molecular geometry and the geometry of crystal structures are also a function of both electric and magnetic displacements, although the manner in which each kind of displacement is involved in geometry determination is dependent on the utilized valence, the resulting interatomic distances and in some cases other atomic orientation effects. Each factor contributes to the probability function of each of the other factors, which makes the geometry situation very complex.
Formulas of Normal Orientation Compounds
That which is referred to as first order normal valence is merely the electric displacement of each element. To make them easier to use, a periodic chart should always be available. The chart form between pages 50 and 51 is most similar to the long form currently in use and incorporates the observed atomic masses based on 12C rather than the natural atomic masses. The 12C system of atomic weights is retained in order to provide a greater degree of familiarity to ease the transition to complete acceptance of the Reciprocal System at all levels of scientific study. For comparison purposes, the atomic weights of the elements are listed in the Appendix using the 16O system of atomic weights which is the natural system on this planet in our galaxy.
Elements of Division One all have positive electric displacements of four or less; the values of electric displacement are the first order positive valences of these elements, x. Similarly, all elements of Division Four have negative electric displacement, the values of which are the first order negative valences of those elements, (x).
In order to form a stable relationship between any two atoms or among several atoms, the net motion between pairs of atoms must be offset or neutralized in one dimension for each atom. This neutralization does not affect the net displacement effects that extend beyond the equivalent space of the involved atomic combination; mass, electric, and magnetic effects. For normal binary compounds this requires only the presence of one kind of element with a normal positive valence and one kind of element with a normal negative valence; for example, between elements involving Divisions I and IV. In order to write the formula of the resulting binary compound, the reasons for the orientation or even what is meant by the word orientation is not necessary to be known, nor is it necessary to know what the resulting interatomic distances will be, to say nothing about what the geometry of the arrangement will be. Only the values of the positive and negative electric displacements of the elements need be used. The general procedures for obtaining chemical formulas for compounds with many elements is not changed other than an understanding of what is theoretically taking place in obtaining the association of the atoms in the various possible arrangements.
Question Set 1
For those who may not be totally familiar with writing formulas of chemical compounds the following exercises are suggested; use the Periodic Chart from page 50 or 51:
-
Using the symbols for the elements of the electric groups 1 and (1), write the formulas for each of the possible compounds by placing the symbol for the electric group 1 element first, followed by the electric group (1) element. For example, LiH, LiCl, KBr, etc.
-
Using the symbols for the electric group 2 elements and the electric group (1) elements, write the formulas for all possible normal valence compounds. Examples: MgF2 , CaCl2.
-
Using the symbols for the electric group 1 elements and the electric group (2) elements, write the formulas for all possible normal valence compounds.
For example: Na2S . K2Se, etc. -
Using the symbols for the electric group 2 elements and the electric group (2) elements, write the formulas for all possible normal valence compounds.
For example: MgO, BaS, etc. -
Using the symbols for the electric group 2 elements and the electric group (3) elements, write the formulas for all possible normal valence compounds.
For example: Mg3N2 , Ca3P2 -
Using the symbols for scandium and titanium with the appropriate symbols for oxygen and sulfur, write the most probable formulas for the binary compounds formed using only normal electric valences. (four formulas)
Other Possible Orientations
The next step in developing the ideas of atomic orientation for chemical compound formation involves differences in the ways in which certain elements may enter orientation; i.e., differences of valence. Relative negativity determines which element is exhibiting which type of valence.
Stating the results of theoretical work, going back over the basics, and giving examples, usually explains the ideas of the different association possibilities for a given representation better than trying to redevelop the thinking from basics to final results.
Representation of the effects of displacement motion in a fixed reference system requires the use of reference points, thereby two linear units of inwardly directed space or time can exist in any given dimension between the +1 of the natural progression in one direction and the +1 of the natural progression in the opposite direction of the same dimension. In a three dimensional reference system in which there can be two linear displacement units between datum points for the outward progression in each of the three dimensions, the number of three dimensionally distributed unidirectional displacement units is 23 = 8, whether those units are represented linearly, 2 units, or rotationally, 8 units.
There are several consequences of the value eight and its representation in a three dimensional Notational Reference Point system. The first is almost obvious; it takes a total of eight (8) electric displacement units to reach an equivalent of zero rotational displacement. A model may help to visualize this situation; consider a circle equally divided into eight segments with edges numbered from the top at zero, clockwise through seven, eight falling on top of the zero.16 Outside of this set of numbers place another set, numbered sequentially in the counter-clockwise direction, again the eight falls on top of the zero. See Appendix Figure VI. To use this device, consider the numbers in one direction around the circle as being positive electric displacements while the numbers in the opposite direction around the circle represent negative electric displacements. There are two 2D axes, and therefore, one magnetic displacement position is worth two electric displacement positions around the circle, but only in the direction chosen as positive displacement. This means that there are only four magnetic displacement units around the valence circle. Magnetic valences are limited to positive numbers with a maximum value of four.
Eight electric displacement units constitute an equivalent zero point or neutral point with respect to balancing the magnitudes of positive and negative displacements against each other to achieve the equivalent of the natural progression in a temporal dimension shared between two atoms. If an atomic component is orienting with a displacement that is displaced from the unit reference, it is using normal valence; if the component is using a displacement from an equivalent zero point, it is said to be using neutral valence.
Another consequence of the equivalence between two linear and eight three dimensionally distributed 1D1dR displacement units is found in the multiple valences exhibited by members of Division IV of the periodic charts. As pointed out in a previous paragraph, one direction around the valence circle model can be thought of as positive displacements while the opposite direction can be thought of as negative displacements. This can be used to determine the exact equivalent positive displacement that an element of Division IV can most readily assume in polyatomic groups with other elements of Division IV which are orienting with normal negative valence. Examples of orientation to an equivalent zero point are found in many compounds such as Cl2O7, SO3, and P4O10. Other examples are also observed in polyatomic radical groups such as sulfate, perchlorate, and nitrate. Division III elements almost exclusively form their binary compounds with Division IV elements by use of the neutral or enhanced neutral orientation although some Division III elements can use magnetic valence in binary compound formation.
Another consequence of this 8R = 2L equivalence can be seen in the shifting to another equivalent zero point two units clock-wise or counter-clockwise around the circle model by either the normal orientation valence or the neutral orientation valence. In order to differentiate, for communication purposes, among the various possible orientations which some atoms can take, valences shifted in the same direction as the normal valence around the circle are referred to as enhanced valences. Valences shifted in the opposite direction of counting on the circle are referred to as diminished valences.
As an example of shifted neutral valence: consider a normal valence of (2) on the valence circle model, shift two more electric positions to (4), then choose the other number, a 4, as the enhanced neutral valence for the element. This is the situation for sulfur in the sulfite radical. Use of enhanced or diminished valence always increases the reactivity of the element or grouping containing such orientations.
Usually valences shifted to a lower positive valence is observed in only one member or atom type in a polyatomic grouping in which the orientation used is neutral or magnetic. Seldom do two atoms of the same element in the same molecule or radical use the same shifted valence, and never to orient toward each other in that structure. One of the atoms in a shifted valence orientation between atoms of the same element must use normal valence; e.g., the thiosulfate radical.
Occasionally, lower negative valence leads to a more symmetrical arrangement of the atoms of the molecule and thus, if the other factors allow for increased probability for stability by such arrangement, it is observed. All elements exhibiting valences greater than two can theoretically engage in the use of diminished valences; however, most shifts lead to decreased probability for stability of the possible resulting compounds as compared to the normal or neutral valences.
Another interesting sidelight on this situation of multiplicity of valence can be seen with respect to the building up sequence of atomic numbers. Disregarding the higher probability for representing nitrogen as 2-2-(3) rather than 2-1-5, this latter representation exhibiting a positive electric displacement of 5 would be expected to show an electric normal positive valence of five. Similarly, sulfur could be written as 2-2-6 rather than the ground state representation of 3-2-(2). We are not in a position by which to state on theoretical grounds which situation actually exists in nature. The possibility exists for an atom to realign the displacements to a less probable “ground state” atomic arrangement to achieve a more probable associational arrangement.
The theoretical possibility of variability of valence for a given element, as shown to exist in the experimentally observed law of multiple proportions, gives further evidence for theoretical validity to the postulates and consequences of the Reciprocal System of theory.
In order to change a particular orientation to a different orientation, sufficient energy must be supplied to separate the atoms in the first orientation and then have the new orientation established which subsequently establishes the new equilibrium distance, force, and geometry. Examples of the application of this may be seen in the oxidation of the sulfide radical to the sulfate radical or in the reduction of the chlorate radical to the chloride radical. Being the equivalent of some opposite orientation does not imply equal energy because the different orientations produce different equilibrium distances between atoms because of the different specific rotations being exhibited and utilized for the different orientations.
In Question Set 2, as pointed out in the column labeled “Type of Orientation” for the example numbered 0, IF5 has enhanced neutral >< normal. This may be symbolized “e.neut. >< n.” and should be read “an enhanced neutral orientation of iodine is oriented in a line with a normal orientation of an atom of fluorine”. Either named element, the central or the peripheral, may be exhibiting the positive orientation and either may be named first. The symbol combination “><” is used to represent the phrase “is oriented in a line with”. An “e.” in combination with “n.” or “m.” is representative of the word “enhanced” and “d.” is for the word “diminished”. Neutral orientations are always from an electric valence and, therefore, the word “electric” is not necessary in that combination. The word “normal” or the abbreviation “n.” by itself may be used to refer to either a negative electric valence, a positive electric valence, or a magnetic valence; the other stated valence indicates the meaning of “n.”. If there could be confusion, an ambiguity can be resolved simply be spelling out the appropriate word. Obviously, a shorthand notation reduces what should be written and said from what is thought.
The word “radical” is used with both monatomic and polyatomic groups to indicate that the atoms involved are not in the elemental form (crystal matrix or molecule), but are associated with some other type of atom or a molecule. Each atom may or may not have an electric charge associated in its structure. Notice that in naming radicals the -ide ending implies only that the named radical is orienting with a negative valence; it is coincidental that most -ide endings are for atomic radicals. The -ate and -ite endings are used with polyatomic groups orienting with net negative valence which have central atoms orienting with valence greater than four, usually some form of neutral valence.
Orientation of atoms and groups of atoms involving hydrogen or elements of the first series of eight elements are somewhat more complex, due to inactive dimensions, than the relatively simple electric and magnetic orientations discussed in this chapter. Organic compounds obviously are in that category and will be discussed later along with some of the interactive characteristics which are dependent on the same factors.
Question Set 2
Will give opportunity for you to test yourself on your understanding of the principles outlined here for possible orientations in a few simple binary compounds. Fill in the requested information:
|
|
Example |
Valence of Peripheral atoms |
Valence of Central atom |
Type of Valence of Central atom |
Type of Orientation |
|
|
IF5 |
(1) |
5 |
enhanced neutral shift 2(8-x) |
enhanced neutral >< normal |
|
7. |
VCl3 |
|
|
|
|
|
8. |
ZnCl2 |
|
|
|
|
|
9. |
Ag2O |
|
|
|
|
|
10. |
NH3 |
|
|
|
|
|
11. |
LaCl3 |
|
|
|
|
|
12. |
BeCl2 |
|
|
|
|
|
13. |
BeH2 |
|
|
|
|
These exercises should have given some experience with writing formulas for the normal electric, neutral, and enhanced neutral orientations for binary compounds involving the Division IV elements with the other Divisions. These exercises have also involved some discussion of other factors in the formation of chemical compounds. Next, we shall consider some binary compounds formed between Division IV elements and Division II elements using magnetic orientation and then extend the discussion to include orientations among polyatomic groups.
Recall the statements in the section on the requirements of atomic orientation that electric and magnetic dimensions are considered separately in the alignment of the dimensions involved in the orientation. For purposes of orientation, the dimensional axes for magnetic displacement are exactly equivalent to the axis of an electric displacement, because orientation is an interaction in dimensional time, not an outside region equivalent of rotational displacement. Whatever the magnetic displacement of a given atom is, that is also a possible positive valence. Thus, the value of the magnetic displacement in each magnetic dimension of an atoms interacting displacement systems may provide the possibility of increasing the probability of having those displacements neutralize an opposite displacement with a smaller number of atoms required in the formula and a more symmetrical spatial arrangement. An XY result is more probable than X2Y or XY2 which is more probable than X2Y3 or X3Y2 and much more probable than larger subnumbers resulting in more complex geometries.
The magnetic displacement represented by the first number of the notation is referred to as the primary magnetic valence, the second number, if different from the first number, is referred to as the secondary magnetic valence. Neither primary nor secondary magnetic valence is more probable than the other, but simplicity and symmetry of spatial geometry for the possible resulting compounds does contribute to the abundance of observed results.
If compounds formed between elements by normal, neutral, “enhanced” normal, or “enhanced neutral” orientations are less probable than the use of magnetic >< normal orientation, then m >< n orientation is what is observed. Magnetic valences can also be enhanced or diminished by the 2L equivalence shift. On the valence circle two electric units equal one magnetic unit when shifting equivalence point. Occasionally, normal magnetic orientation is less probable than a “diminished magnetic” >< normal orientation, in which case the “d.m.” >< n. orientation is observed. A “diminished magnetic” valence is one numerical unit less than the normally observed “magnetic” valence, while an “enhanced magnetic” valence is one numerical unit greater than the highest normal magnetic valence. These variations result from dimensional contributions to probability calculations. A positive “diminished electric” valence is highly improbable due to the availability of the magnetic valences.
There are some examples of situations in which a diminished valence has very low probability for stability when considered alone, but when combined with a normal, either magnetic or electric, orientation molecule formed by the same elements, geometric considerations provide the diminished valence orientation sufficient stability for a combined existence with the normal orientation molecule.
Question Set 3
Use the previously given column heading set-up for exercises in Question Set 2. Continue that exercise incorporating the use of magnetic valences along with the others while completing the requested information about each of these compounds:
|
14. |
SO3 |
|
|
|
|
|
15. |
SO2 |
|
|
|
|
|
16. |
As2O5 |
|
|
|
|
|
17. |
As2O3 |
|
|
|
|
|
18. |
IF7 |
|
|
|
|
|
19. |
MnCl3 |
|
|
|
|
|
20. |
Mn2O7 |
|
|
|
|
|
21. |
VF3 |
|
|
|
|
|
22. |
VF4 |
|
|
|
|
|
23. |
VF5 |
|
|
|
|
|
24. |
FeBr2 |
|
|
|
|
|
25. |
C3O2 |
|
|
|
|
|
(Hint – combine two possible valences) |
|||||
|
26. |
KClO3 |
|
|
|
|
|
27. |
K2SO4 |
|
|
|
|
A negative valence hydrogen atom is referred to as the normal electric valence. Hydrogen exhibiting a positive valence is using a magnetic valence since there is no positive electric displacement that can be used for a normal positive electric orientation. Hydrogen is always positive to the other non-metals and negative to any element using positive electric or magnetic orientations.
Intermixed solvent molecules and radicals from crystal structures form solutions by orienting their constituents based strictly on the same rules of orientation used for the pure substances. Consider the representation of the substance water; H2O. The oxygen is obviously the central atom, and being the more electronegative of the two elements present, must exhibit and orient for molecule formation with its normal valence of (2), electric negative two. The hydrogen must therefore orient its magnetic displacement in a temporal line so as to counterbalance one of the negative electric displacements of the oxygen. Notice that each hydrogen atom has only one effective magnetic displacement and, thereby, one of its magnetic dimensions is ineffective, leading to inactive dimensions in its compounds. These inactive dimensions respond to externally applied magnetic fields in a different manner from that of merely unequal magnetic displacements in the magnetic dimensions of other elements. In water each hydrogen atom has its negative electric displacement directed in three dimensional time such that it can orient with any atom or radical orienting with positive valence, whether as an electric, magnetic, or neutral orientation. Of the subsequent solvent-solute orientations, electric to electric orientation leads to interatomic forces that are somewhat more mobile with respect to thermal considerations than corresponding electric to magnetic orientations. Magnetic orientations always leave positive electric displacements unneutralized leading to solvate formation. Valence Group I chlorides do not crystalize from water solution incorporating the water in the crystal structure. Most Division II chlorides do, while only some of the Division III chlorides do; for example, Iron II chloride does, as does zinc chloride and copper II chloride, but not copper I chloride, to name just four examples. Hydrate formation is not strictly a dipole effect, although polarization effects for molecules is related to the types of orientation present in the molecules.
The orientations involved in the elemental forms, both metallic and non-metallic, is similar, but with significant differences that involve extended geometric relationships not bounded by the limitations imposed by negative electric displacement.
What About Electric Charges?
Notice that electric charges have not been mentioned in describing the orientation of atoms in any compounds thus far considered. The examples used range through the various types of compounds as classified according to their behavior in water solutions, along with some thermal behavior. The question of whether the substance acts as an electrolyte in water solution has nothing to do with the way in which the atoms are oriented in the pure forms of the substances, whether solid, liquid, or gas. The presence of charged radicals, which are referred to as ions, is often questionable, although in some situations ions are definitely present, particularly in gas phase situations.
Relative conductivity of solutions is a matter of whether the resulting structures can be caused to take on an electric charge, and if so which structures can be most readily caused to do so, with the applied source (E.M.F.) of available motion (charged or uncharged electrons). Reorientation effects may result from the relative magnitudes of charge effects, thermal effects, and the various possible orientations. The source of the electromotive force and its magnitude determines the nature of the resultant effect. Which structural representations in dimensional space can be caused to take on an electric charge, to what magnitude, and what different orientations are stable under the new conditions are the principal observed results.
The initial orientation of the atoms and groups in the pure substances has nothing to do with any subsequently caused reorientation within those structural groups other than what is available and contributing to the total energy situation. Conducting the electric current always causes a series of motion interchanges which may or may not result in a net orientational change for the atoms of the initial substances.
In the liquid and solid phases of matter the association of solvent molecules with solute radical atoms or polyatomic groups is within unit space, and therefore, any electric charge present causes the grouping in the contiguous space units to exhibit an induced opposite charge which cancels the measurability of the presence of electric charge in any given sample of solid or liquid, whether of a pure substance or a mixture of different molecular and radical species.
Most phases of Physics and all areas of Chemistry involve atoms in some kind of associational pattern. Thus, it is reasonable to consider the concepts of atomic orientation as basic information appropriate for all other specific topics. Other concepts and relationships of atomic structure appropriate to other specific topics of discussion are addressed in the appropriate context. Results found in one area of investigation apply directly in all other areas with assurance of complete compatibility for results of investigations within this theoretical system because of deduction from a single premise. Extensive detailed explanations are often omitted in one area of discussion since at some other point a complete and detailed development of the phenomena is given.
