CHAPTER 20
Chain Compounds
In undertaking a general survey of such an extended field as that of the structure of the organic compounds it is obviously essential to use some kind of a classification system to group the compounds of similar characteristics together, so that we may avoid the necessity of dealing with so many individual substances. The distinction between chain and ring compounds has already been mentioned. The chemical properties of the chain compounds are determined primarily by the nature of the positive and negative radicals or atoms, and it will therefore be convenient to set up two separate classifications for these compounds, one on the basis of the positive component, and the other on the basis of the negative component. In general, the classifications utilized in this work will conform to the commonly recognized groupings, but the defining criteria will not necessarily be the same, and this will result in some divergence in certain cases.
The first positive classification that we will consider comprises those compounds whose positive components contain valence four carbon atoms. These are called paraffins. This name originally referred only to hydrocarbons, but as used herein it will apply to all chain compounds with valence four carbon at the positive end of the molecule. The term “saturated compound” is commonly used with essentially the same significance so far as the chain compounds are concerned, but its application is usually extended to the cyclic compounds as well. To avoid confusion it will not be used in this work, since the cyclic compounds cannot be considered saturated on the basis of the criteria that we are setting up. The paraffin hydrocarbon, or alkane, chain is a linking of CH2 neutral groups with a CH2 positive radical at one end of the chain, and a negative hydrogen atom at the other. The cohesion between this hydrogen atom and the adjacent CH2 group is very strong, and for most purposes it will be convenient to regard the CH2•H combination as a negative CH3 radical. On this basis, the paraffin hydrocarbon chain is CH3•(CH2)n•CH3.
If a valence two carbon atom is substituted for the valence four carbon atom of the paraffins, the result is an olefin, a chain which is identical with that of the paraffins except that it has the primary magnetic valence radical CH instead of the normal valence radical CH3 in the positive position. The general formula for the olefin hydrocarbons, or alkenes, is CH•(CH2)n•CH3.
In the usual version of this formula one of the CH2 groups is placed outside of the CH group, but this is obviously incompatible with the structural principles developed in the preceding pages. On first consideration it might appear that the chemical evidence is favorable to the conventional CH2•CH sequence. When we remove all of the internal magnetic neutral groups we come down to CH•CH3 as the theoretical structure of ethylene, the first of the olefins, whereas it is generally agreed that the chemical behavior of this compound is more in harmony with the structure CH2•CH2. This apparent contradiction is explained by the nature of the CH3 negative radical. As has been pointed out, this radical is actually CH2•H. For most purposes the combination may be treated as a single unit, but if we express the ethylene formula in full form as CH•CH2•H it can be seen that the association between the CH and H structural units is closer than that between CH2 and H. It is true that the CH2 group is between CH and H when the ethylene molecule is intact, but CH and H are partners in a valence equilibrium, whereas the intervening CH2 group is neutral. Consequently, if the molecule is sufficiently disturbed by chemical or other means, the CH and H units join and the compound enters the subsequent reaction as two methylene (CH2) molecules. This is not an unusual situation. Many observers have commented that the reacting molecule under such circumstances is not necessarily the same as the static molecule.
A valence one carbon atom in the positive position produces an acetylene. Both the olefin and acetylene classifications, as herein defined, should be understood as including all compounds with the specified positive components, not merely the hydrocarbons. In the acetylenes, as in the olefins, the currently accepted molecular formulas must be revised to put the positive valence component at the end of the chain. We also find that the valence one orientation of a lone carbon atom is more stable if it is joined to a neutral group in which carbon has the same valence, rather than to one in which the carbon valence is +2. The independent carbon atom that constitutes the positive component of the acetylenes is therefore followed by a CH neutral group. The remainder of the acetylene hydrocarbon, or alkyne, molecule is identical with the corresponding portion of a molecule of either of the other two hydrocarbon chains, and the general formula is C•CH•(CH2)n•CH3. Acetylene itself is similar to ethylene in that the true structure is C•CH•H2 with a valence equilibrium between the single C and H atoms which causes them to combine if the molecule breaks up. The compound therefore acts chemically as two CH units.
Addition of CH2 neutral groups to the straight chain hydrocarbons does not necessarily take place in the existing chain. The incoming groups may instead be inserted between the positive and negative components of any of the neutral groups, enlarging that group from CH2 to CH•CH2•H2 which we may write as CH•CH3, or CHCH3, as previously indicated. Further additions may then be made in the same manner as they are made in the principal chain, lengthening the neutral group indefinitely. Such a lengthened group is known as a branch of the principal chain, and structures of this kind are called branched chain compounds.
No branching of the CH3 radical is possible, since addition of a CH2 group results in CH2•CH2•H2 or CH2•CH3, which merely extends the straight chain. A CH2 group may be added to the CH olefin radical however, as the product in this case is CCH3, which is not equivalent to an extension of the chain. This CCH3 group may then be lengthened in the usual manner to C•CH2•CH3, and so on.
Under the accepted systems of nomenclature the branched chain compounds are named as derivatives of the straight chain compounds, the chain position being indicated by number, as in 2-methyl butane, 2,3-dimethyl hexane, etc. The added possibility of a modification of the positive radical in the olefins introduces an extra variation into the system which is taken into account by setting up several basic classifications: 1-olefins, 2-olefins, 3-olefins, and so on. Branching is handled in the same manner as in the paraffins, and the compounds have names such as 2-ethyl-1-hexene, 3,4-dimethyl-2-pentene, etc.
The names applied to the paraffins under this current system are equally applicable to these compounds on the basis of the structural relations developed in this work. However, the current ideas as to the structure of the olefins and acetylenes, and the system of nomenclature that has been applied to them, are products of the electronic theory of compound formation. The results of our theoretical development show that certain modifications of the previously accepted structural arrangements are required, as has been noted, and the nature of these modifications is such that changes in the names applied to some of the compounds would also be appropriate. On this new basis no special system of names is required for the olefins, as the paraffin system can be applied to the olefins as well. The only difference between the two is in the branching of the olefin radical, and this can be handled by utilizing the 1-alkyl term, available but not used in the paraffin compounds. On this basis 1-pentene, CH•(CH2)3•CH3, will become simply pentene, while2-pentene, CCH3•(CH2)2•CH3, becomes 1-methyl butene, and 3-pentene, (C•CH2•CH3)•CH2•CH3, becomes 1-ethyl propene. The paraffin names are also applicable to the acetylenes in the same manner. 1-pentyne, C•CH•(CH2)2•CH3, becomes pentyne; 2-pentyne, C•CCH3•CH2•CH3, becomes 2-methyl butyne, and so on. Such a revision of the nomenclature is not only desirable from the standpoint of more accurately reflecting the true structure of the molecules, and for the sake of uniformity, but also accomplishes a substantial amount of simplification.
The information derived from theory will likewise require some modification of the conventional methods of representing the molecular structure of the organic compounds. The so-called “extended” formulas, based on concepts such as electrons and double bonds that have no place in the molecule as we find it, must be discarded. But for most purposes the exact arrangement of the individual atoms is immaterial. The structural unit is the group rather than the atom, and the positions of the groups determine the nature and magnitude of the structure-dependent properties of the compound. The notation that has been used thus far, the “condensed” structural formula which shows only the composition and sequence of the groups, is therefore adequate for most normal applications.
The usual arrangement of these condensed formulas is not entirely satisfactory, as it does not recognize the existence of positive and negative valences, and therefore fails to distinguish between groups of the same composition but opposite valence. The CH3 end groups in the paraffin molecule, for example, are currently regarded as identical. Since the opposing valences play a very important part in the molecular structure it is desirable that the formula should definitely indicate the positive and negative components of the compound. This can be accomplished without any serious dislocation of familiar patterns by identifying the positive and negative components of the compound as a whole with the left and right ends of the formula respectively, as is common practice in the inorganic division.
It would be logical to extend this policy to the individual components of the molecules, and that probably should be done some day as a matter of consistency, but some compromise with logic and consistency seems advisable in this present work in order to avoid creating further complications for the readers, who already have many unavoidable departures from conventional practice to contend with. The familiar expressions for such primary units as NH2 and OH will. therefore be retained, together with expansions such as NH•CH2•CH3, O•CH2•CH3, etc., even though this reverses the regular positive to negative order in most of the negative radicals. Continued use of CH3 rather than CH2•H to represent the negative methyl radical is also a departure from consistent practice, but in this case the condensed form is not only more familiar but also more convenient. The full CH2•H representation will therefore be used only where, as in the discussion of the structure of the ethylene molecule, it is necessary to stress the true nature of the radical. In the case of the analogous CH2 negative radical there is no significant advantage to be gained by use of the condensed expression, and this radical, which is a combination of a CH neutral group and a negative hydrogen atom will be shown in its true form as CH•H.
For a correct representation of the molecular structure it is essential that the neutral groups be clearly identified. Where there are methyl substitutions, the identification can be accomplished by omitting the dividing mark between the components of the neutral group; e.g., CH3•CHCH3•CH2•CHCH3•CH3, 2,4-dimethyl pentane.Longer neutral groups can be identified by parentheses, the positive-negative order being preserved within the group. The formula of 3-propyl pentane on this basis is CH3•CH2•(CH•CH2•CH2•CH3)•CH2•CH3.If further subdivision within the neutral groups is necessary, the distinction between main and subgroupings can be indicated by brackets or other suitable symbols.
Where a valence two negative component is involved and the chain is double, the customary expression such as (CH3•CH2)2•O is appropriate if the chains are equal. Unequal chains can be represented by treating the valence two component and one of the branches as a negative radical in this manner: CH2•CH2•CH2•(O•CH2•CH3),or the two branches can be shown on separate lines, as
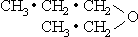
In order to facilitate the presentation of the new principles of molecular structure that have been developed from the postulates of the Reciprocal System the revised structural formulas as described in the foregoing paragraphs will be used throughout this work. In designating positions in the chain we will number from the positive end, rather than following the Geneva system, which regards the two ends as interchangeable. The different numbering is necessary for clarity, in view of the modifications that have been made, not only in the order of the groups but also, in some cases, in the group composition. However, this revised numbering will be used only for purposes of the discussion, and the accepted names of the compounds will be retained, to avoid unnecessary confusion. A complete overhaul of the organic nomenclature will be advisable sooner or later.
The somewhat minor modifications of current structural ideas that are required in the olefins and acetylenes become more significant in the diolefins, a class of compounds in which a pair of CH neutral groups with the acetylene carbon valence (one) is inserted into the olefin chain, a valence two structure. The C5 compounds of this class are known as pentadienes. If the CH groups replace the CH2 groups in the third and fourth positions of pentene the result is CH•CH2•CH•CH•CH3.
Instead of using the same numbering system that is applied to the other hydrocarbon families, the diolefins are numbered according to the locations of the hypothetical “double bonds,” and this compound is called 1,3-pentadiene. Since the CH3 group at the negative end of the pentene molecule is actually CH2•H2 the CH2 portion is open to replacement by CH. The incoming CH groups may therefore occupy the fourth and fifth positions, producing CH•CH2•CH2•CH•CH•H2 now called 1,4-pentadiene. Another possible structure involves removing the hydrogen atom from the CH positive radical, and splitting the molecule into two chains. If the chains are equal, we have C(CH•CH3)2, which we may also represent as
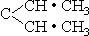
This is 2,3-pentadiene. A variation of this structure removes the CH2 group from one of the CH3 combinations. This reduces the compound to a C4 status, but it can be brought back up to a pentadiene by inserting the CH2 group in the other branch, which produces what is called 1,2-pentadiene:
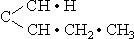
One of the most important of the diolefins, from the industrial standpoint, is isoprene, another C5 compound, currently called 2-methyl1,3-butadiene. The structure is the same as that of 1,4-pentadiene, except that the CH2 group next to the first of the CH neutral groups is moved out of the chain and attached to the CH group as a branch: CH•CH2•CCH3•CH•H.
Nitrogen, which is next to carbon in the atomic series, is also the next most prolific in the formation of compounds. Some of the “carbon” compounds, such as urea, one of the first organic compounds to be synthesized, actually contain more nitrogen than carbon, but the positive component in these compounds is carbon, and the lengthening of the chain takes place primarily by the addition of carbon groups. There are other compounds, however, in which nitrogen takes the positive role both in the compound as a whole and in the neutral groups.
Corresponding to the hydrocarbons are the hydronitrogens. The positive nitrogen radical in these compounds is NH2+, in which nitrogen has the enhanced neutral valence three. A combination of this radical with the negative amine group is hydrazine, NH2•NH2. Inserting one NH neutral group we obtain triazane, NH2•NH•NH2. Another similar addition produces tetrazane, NH2•NH•NH•NH2. Just how far this addition process can be carried is uncertain, as the theoretical limits have not been established, and the hydronitrogens have not been given the same exhaustive study as the corresponding carbon compounds. A nitrogen series corresponding to the acetylenes has a lone nitrogen atom with the secondary magnetic valence one as the positive component. The parent compound of this series is diimide, N•NH2. One added NH neutral group results in triazene, N•NH•NH2, and by a second addition we obtain tetrazene, N•NH•NH•NH2. Here again, the ultimate length of the chain is uncertain.
All of the neutral groups in these nitrogen compounds have the composition NH2 in which nitrogen has the secondary magnetic valence one. A neutral group NH2 based on the primary magnetic valence is theoretically possible, but this group is identical with the amine radical except for the rotational orientation, and the orientation is subject to change in accordance with the relative probabilities. The amine radical is a more probable structure, and it prevents the existence of the NH2 neutral group.
The NH2+ radical is also a much less probable structure than the amine radical, in which nitrogen has its normal negative valence, but this positive radical is not in competition with the amine group. Wherever a number of NH2 units exist in close proximity the inter-atomic forces tend toward combination, and in order that such combination may take place some groups must be reoriented so that they may act as the positive components of the compounds. The NH2+ radical has the most probable of the positive orientations, and it therefore takes over the positive role in NH2•NH2 and similar combinations, a position that is not open to the amine radical. The NH2 neutral group has no such protected status.
Beyond carbon and nitrogen the ability to form compounds of the molecular type drops sharply, but the corresponding elements in the higher groups do participate in a few compounds of this nature. Silicon forms a series of hydrides analogous to the paraffin hydrocarbons, with the composition SiH3•(SiH2)n•H, and also some compounds intermediate between the silicon and carbon chains. Typical examples of the latter are Si3•CH2•SiH2•H, and Si(CH3)3•CH2•SiH2•CH2•SiH2•H. Germanium forms a series of hydrides, known as germanes, which are similar to the silicon hydrides, or silanes, and have the composition Ge3•(GeH2)n•H. Only a few members of this series are known. An unstable tin hydride, Sn3•SnH2•H2 has also been reported. It could be expected that the higher valence three elements would form a limited number of compounds similar to the hydronitrogens, but the known compounds of this type are still scarce. Among those that have been reported are diphosphene, PH2•PH2, and cacodyl, As(CH3)2•As(CH3)2. Since the minimum magnetic valence of phosphorus and arsenic is two, these compounds cannot have the hydrazine structure NH2•NH•H2 and are probably PH•PH2•H and AsCH3•As(CH3)2•CH3. As pointed out in connection with ethylene and acetylene, the chemical behavior of such compounds is explained by the tendency of the positive and negative components of the compound as a whole, such as PH and H in diphosphene, to join when the compound is disturbed during a chemical reaction.
Another series of compounds of the molecular class, but not related to either carbon or nitrogen, is based on boron. Because it acts as a Division IV element in these two-dimensional compounds, boron takes the valence five, rather than the normal valence three which it has in a compound such as B2O3, where it acts as an element of Division I. The valence one radical on the valence five basis would be BH4, or an equivalent, but such a radical would be three-dimensional, and not capable of joining a two-dimensional chain. The positive radical in the boron chain is therefore the valence two combination BH3. As in the hydrocarbons, the negative component of the molecule as a whole is hydrogen, and because of the valence of the positive radical two negative hydrogen atoms are required. Here again, the association between the hydrogen atoms and the adjacent BH neutral group is close, as in the hydrocarbons, and the combination could be regarded as a valence two negative BH3 radical. For present purposes, however, it appears advisable to show it in its true form as BH•H2.
The magnetic neutral groups of the boron compounds can be formed on the basis of either the primary or the secondary magnetic valence, which produce BH2 and BH respectively. Because it minimizes the number of hydrogen atoms at the negative end of the molecule, the negative radical BH•H2 takes precedence over BH2•H2 even where the interior groups are BH2 combinations. This presence of a BH neutral group at the negative end of the compound, together with some other factors that apparently favor BH over BH2, has the effect of making the BH structures more stable than those in which the neutral groups are BH2.
The basic hydride of boron is diborane, BH3•BH•H2. Addition of BH neutral groups produces a series of compounds with the composition BH3•(BH)n•H2, the best known of which are hexaborane, in which n is 5, and decaborane, in which n is 9. Substitution of a pair of BH2 groups for two of the BH groups results in a series which has the composition BH3•(BH2)2•(BH)n•H2. Beyond tetraborane, the first member of this series (n=1),these compounds, as indicated in the preceding paragraph, are less stable than the corresponding compounds of the all-BH series. In all of these boron compounds replacement of hydrogen atoms by other valence one atoms or radicals is possible in the same manner as in the hydrocarbons, but to a much more limited extent.
As noted earlier, the extension of Division IV characteristics into Division III, which gives rise to the two-dimensional combining tendencies of boron, does not apply to the corresponding elements of the higher groups to any substantial degree, and they do not duplicate the boron series of compounds. There is an unstable hydride of aluminum, Al2H6, and a compound Ga2H6 called digallane, both of which may be structurally similar to diborane, but there is little, if any lengthening of these compounds by means of magnetic neutral groups.
From the overall chemical standpoint, the molecular compounds formed by positive elements other than carbon are not of much concern, and they are given little or no attention in any but specialized textbooks. They are important in the present connection, however, because they serve to confirm the theoretical conclusions that were reached with respect to the structure of the carbon compounds. The nitrogen and boron compounds are not only constructed in accordance with the general pattern deduced from theory, and followed by the carbon compounds-that is, a chain of magnetic neutral groups with a positive radical at one end and a negative radical at the other-but also support the theoretical conclusions with respect to the structural details, inasmuch as they are like the carbon compounds in those respects in which the theory finds these elements to be alike, whereas they differ from the carbon compounds in those respects in which there are theoretical differences. For example, all three of these elements form both valence two (CH2 etc.) and valence one (CH etc.) magnetic neutral groups (with the exception of NH2, the absence of which has been explained), because these magnetic valences are properties of the group of elements (2A) to which all three belong. On the other hand, the radicals in the end positions are unlike because the electric valences, which apply to these radicals, are properties of each of the three elements individually, and they are all different.
The second system of classification of the organic chain compounds, that based on the nature of the negative components, is not an alternate but a parallel system. A compound classified as an alcohol because of the nature of its negative component also belongs to one of the categories set up on the basis of the identity of the positive component. The previous discussion was confined mainly to the hydrocarbons to simplify the presentation, but all of the statements that were made with reference to compounds in which the negative component is hydrogen, alone or in combination with CH2 as a negative CH3 radical, are equally applicable to those in which the hydrogen has been replaced by an equivalent negative atom or group. Thus we have paraffinic alcohols, olefinic (unsaturated) alcohols, and so on.
The primary requirement for the one for one substitutions is that the valence of the substituent must conform to the hydrogen valence both in magnitude and in sign. This requirement has been obscured to a large extent by current structural theories which do not recognize the existence of positive and negative valence in organic compounds, but some of the hydrogen atoms in these compounds are positive and others are negative, and this determines what substitutions can take place. Hydrogen in combination with carbon is negative, and may be replaced by any of the halogens or by negative radicals. Hydrogen combined with oxygen is positive, and can therefore be replaced only by positive elements and radicals. Thus from acetic acid, CH3•CO•OH, we obtain by substitution CH2Cl•CO•OH, chloroacetic acid, but CH3•CO•ONa, or Na•(O•CO•CH3), sodium acetate.
A hydrogen atom acting alone may be either positive or negative, depending on its environment. The hydrogen atom at the end of a hydrocarbon chain is negative, and may be replaced by a halogen. CH3•CH2•H, ethane, becomes CH3•CH2•Cl, ethyl chloride. The lone hydrogen atom in formic acid, H•CO•OH, is positive, and a halogen cannot replace it. The normal valence alkali elements cannot replace this lone magnetic valence hydrogen atom either, and an incoming positive atom goes to the OH radical. The hydrogen in N-H combinations is also resistant to monatomic substitutions, but replacement by radicals of the proper valence is readily accomplished.
Elements with higher valences substitute quite freely for either carbon or hydrogen in the positive and negative radicals, but enter into the magnetic neutral groups mainly as constituents of the common valence one radicals: OH, NH2, etc. Except in the direct carbon-oxygen combination CO, a single atom of valence two or three in a neutral group is necessarily a constituent of an extended radical such as (O•CH2•CH3).
In beginning a consideration of the principal families of substituted compounds, we will look first at the alcohols. This alcohol classification is one of several which result from the addition of oxygen to the hydrocarbons in different ways. Here an OH radical is directly attached to a hydrocarbon group, replacing a negative hydrogen atom. It is not essential, however, that this OH group replace the particular atom that constitutes the negative component of the compound as a whole. The chemical behavior of the normal alcohols, in which the OH radical is at the end of the chain, as in ethyl alcohol, CH3•CH2•OH, is closely paralleled if OH is substituted for a hydrogen atom in one of the neutral groups, as in secondary butyl alcohol, CH3•CH2•CHOH•CH3. If the substitution takes place in the positive radical the result is somewhat different. Such a substitution is more readily made if oxygen is first introduced at the more favorable negative end of the compound, and the product of a double OH substitution is a dibasic alcohol, or glycol, the most familiar compound being ethylene glycol, CH2OH•CH2•OH.
Earlier in this chapter it was noted that the paraffin hydrocarbons are not actually the symmetrical structures that they appear to be. There is a combination of one carbon atom and three hydrogen atoms at each end of the molecule, but one end of the chain is necessarily positive, which means that the CH3 group at this end is a radical in which carbon has the +4 valence, while the other end is necessarily negative, and this, as previously explained, means that the CH3 group in this position is actually a close association of a negative hydrogen atom with a CH2 neutral group in which carbon has its +2 valence. Where the true molecular structure is important, as in understanding the chemical behavior of ethylene, it is essential to recognize that CH3 in the negative position is, in fact, CH2•H. As indicated in the formula given for ethylene glycol, this same asymmetry also exists in the other seemingly symmetrical compounds. The CH2OH group in the positive position in the glycols has a +4 carbon valence and a group valence of +1 . In the negative position, the carbon valence is +2, and the true structure is CH2•OH. The chemistry textbooks contain statements such as this: “Theoretically the simplest glycol should be dihydroxy methane, CH2(OH)2.” The foregoing explanation of the glycol structure shows why this compound would not be a glycol, and why no such compound has been found.
An oxygen atom added to a hydrocarbon may replace the two hydrogen atoms of a CH2 neutral group rather than forming an OH radical. The resulting group CO is very close to the point of not being able to act as a magnetic neutral group at all, and it is greatly restricted as to its position in the molecule. Straight chains of CO groups similar to the CH2 chains are not possible. This explains why carbon monoxide occurs as a separate compound, whereas methylene does not. In order to enable the CO group to join an organic combination some assistance from the geometric arrangement is necessary (a point which will be discussed further in connection with our examination of the ring compounds), and in the chain compounds this can be accomplished most readily at the negative end of the molecule. In the usual arrangement, therefore, a single CO neutral group is joined directly to the negative atom or radical.
If the negative component is the radical OH, the resulting compound contains the combination CO•OH, and is an acid. Acetic acid, CH3•CO•OH, and acrylic acid, CH•CH2•CO•OH, are representative paraffinic and olefinic (unsaturated) acids respectively. Here again, a shift of the carbon valence to +4 produces a positive radical of the same composition, and enables formation of dibasic acids, such as oxalic acid, COOH•CO•OH, maleic acid, COOH•CH•CH•CO•OH, etc.
Modification of the acid structure by substituting an alkyl group for the hydroxyl hydrogen results in another prolific family of compounds, the esters. Ethyl acetate, CH3•CO•(O•CH2•CH3), and diethyl oxalate, CO(O•CH2•CH3)•CO•(O•CH2•CH3), are typical of the mono and diesters respectively. A similar substitution in an alcohol produces an ether. This compound may be considered as a radical of the composition O•(CH2)n•CH3 in combination with an alkyl group. If we now substitute a second radical of the same kind for one of the hydrogen atoms in the adjacent hydrocarbon group we obtain an acetal. Another such replacement results in an orthoester. By successive substitutions in ethyl alcohol, CH3•CH2•OH, for instance, we produce methyl ethyl ether, CH3•CH2•(O•CH3), dimethyl acetyl, CH3•CH•(O•CH3)2, and trimethyl orthoacetate, CH3•C•(O•CH3)3. Elimination of a water molecule from two acid molecules produces an anhydride, such as acetic anhydride, (CH3•CO)2•O. No new structural features are involved in these compounds.
If the CO neutral group is joined directly to the negative hydrogen atom at the end of the hydrocarbon chain the compound is an aldehyde. Acetaldehyde, CH3•CO•H, is the most familiar member of this family. The aldehyde radical is usually expressed as CHO (to avoid confusion with the OH radical, the textbooks say), but this does not reflect the true status of the CO combination as a neutral group. It may be worth noting that the CHO representation also does not explain, as the CO•H formula does, why one of the most prominent features of the aldehydes is that they are good reducing agents. Like the other organic families that have been discussed thus far, the aldehydes form dibasic, as well as monobasic, compounds. The simplest dibasic aldehyde is glyoxal, COH•CO•H. As in such structures as COOH•CO•OH, the conversion of the negative radical to a positive radical involves a valence shift, but in the acids the change is in the carbon valence, which goes from +2 in CO•OH to +4 in COOH, while in the aldehydes the change is in the hydrogen valence, which goes from -1 in CO•H to +1 in COH.
These are the most basic valence changes in organic reactions, and their concurrent accomplishment is an essential element in a wide variety of chemical reactions. For instance, in the addition reactions that convert olefinic compounds to the paraffin status, such as adding HBr to acrylic acid, the carbon valence in the positive radical increases two units from +2 to +4. At the same time, the hydrogen atom that had a +1 valence in HBr decreases that valence by two units to the -1 level in the addition product CH2Br•CH2•CO•OH. There are no obstacles in the way of a change of valence. This is merely a matter of reorientation, a change of rotational direction, and each atom is free to reorient itself to conform tc its environment. But the positive-negative balance in the compound must be maintained, and the change from positive to negative, or vice versa, in the hydrogen valence is one of the most common ways of compensating for an increase or decrease in the carbon valence.
Because of the close association between the negative hydrogen atom of the hydrocarbons and the adjoining CH2 group, the CO neutral group is able to occupy a position adjoining the CH2•H combination as an alternate to the aldehyde position next to the hydrogen atom. In this more remote position it is near the limit of stability, and this makes association with the positive radical more probable than participation in the negative combination CO•CH2•H. For this reason, the monobasic compounds in this family, the ketones, have oxygen in the positive radical, COCH3, rather than in the negative radical as usual. The first member of the family, dimethyl ketone, or acetone, has the structure COCH3•CH2•H. The corresponding dibasic compound is dimethyl diketone, COCH3•CO•CH2•H.
The monobasic ketone structure can be verified by comparing the results of simple addition reactions of the ketones with those of the aldehydes, the isomeric compounds in which the CO group is neutral. The addition of hydrogen to the aldehydes proceeds in this manner:
CH3•CH2•CO•H + H2 = CH3•CH2•CH2•OH
The final product, propyl alcohol, is a normal chain compound with a CH3 radical in the positive position, just as in the aldehyde itself. Only the negative end of the molecule has been altered. If the CO group in the corresponding ketone, methyl ethyl ketone, or 2-butanone, had the same status as in the aldehyde (that is, if the compound were CH3•CH2•CO•CH3), we would expect essentially the same result. We would expect the CH3 positive radical to remain intact, and the product to be a primary, or perhaps a secondary, alcohol. But since the CO group in the ketone is part of a radical in which the carbon valence is four, and the compound is actually COCH3•CH2•CH3, both CH3 groups are negative. Addition of a hydrogen atom to the neutral group CH2 produces a third negative CH3 group. Inasmuch as no positive CH radical is present, hydrogenation results in a tertiary alcohol, in which the CH3 groups are negative, as in the original ketone:
COCH3•CH2•CH3 + H2 = C(CH3)3•OH
In the organic chain compounds thus far discussed, lengthening of the chain is accomplished mainly by the addition of CH2 neutral groups and, in some cases, CH•CH pairs. Introduction of oxygen produces a neutral group CHOH, and substitution of this group for CH2 originates additional families of compounds. These include such important substances as the hydroxy acids, the polyhydroxy alcohols, and the saccharides. The hydroxy acids may be either monobasic, like lactic acid, CH3•CHOH•CO•OH, or dibasic, similar to tartaric acid, COOH•(CHOH)2•CO•OH. In both cases the chains can be extended by adding more CHOH groups, although addition of CH2 is also possible, as in malic acid, COOH•CHOH•CH2•CO•OH. The polyhydroxy alcohols are extensions of the glycol chain with CHOH neutral groups. The general formula is CH2OH•(CHOH)n• CH2•OH. The saccharides result from conversion of the CH3 radicals in the aldehydes and ketones to CH2OH and addition of CHOH neutral groups. The products derived from the aldehydes are aldoses, the general formula for which is CH2OH•(CHOH)n•CO•H. Those derived from the ketones are ketoses, and have the structure (CO•CH2•OH)•(CHOH)n•CH2•OH.
When nitrogen is introduced into an aldehyde or ketone, replacing the carbon-oxygen combination with a triple combination of nitrogen, hydrogen, and oxygen in the form of the valence two oxime radical NH•O, the nature of the addition products again shows the same relation to the structures of the two oxo derivatives that we noted in the case of hydrogen addition. Adding NH to the aldehyde alters only the negative radical, which expands from CO•H to CH•NH•O. Propionaldehyde, CH3•CH2•CO•H, for example, becomes propionaldehyde oxime, CH3•CH2•(CH•NH•O). On the other hand, addition of NH to the ketones requires a molecular rearrangement to bring both CH3 groups, which are negative, into combination with positive carbon in the positive radical. Adding NH to acetone, COCH3•CH3 produces dimethyl ketoxime, C(CH3)2•NH•O. As indicated in these formulas, it is necessary to change the expression for the oxime radical from the conventional NOH to NH•O to show the true composition.
Another way in which nitrogen may be introduced into the hydrocarbons is by substituting the NH2 amine group for negative hydrogen. Further substitutions are then possible for the positive hydrogen atoms in NH2, giving rise to a great variety of structures. The compounds in which the NH2 radical remains intact are primary amines, those with NH and one positive substitution are secondary amines, and those in which both hydrogen atoms have been replaced, leaving only the lone nitrogen atom from the original amine group, are tertiary amines. Since the amine replacements are positive, these compounds may have more than one olefinic branch, as in diallyamine, (CH•CH2•CH2)2•NH, a type of structure not found in the hydrocarbons, where all hydrogen atoms are negative, and can be replaced only by negative substituents. Diamines have the usual double structure, with CH2NH2 in the positive position and the normal amine combination CH2•NH2 at the negative end of the molecule.
Like the hydroxyl group OH which attaches to CH to form the neutral group CHOH, the amine group joins with CH to form a neutral group CHNH2. This group is more restricted as to its position in the chains than CHOH, which substitutes quite freely for CH2, but it has a special importance in that it is an essential component of the amino acids, which, in turn, are the principal building blocks of the proteins, the basic constituents of living matter. In the monoacids the CHNH2 group in effect extends the acid radical from CO•OH to CHNH2•CO•OH. Further lengthening of the chain takes place by addition of hydrocarbon neutral groups, or CHOH, rather than CHNH2. Thus d-alanine, CH3•CHNH2•CO•OH lengthens to 1-leucine, CH3•CHCH3•CH2•CHNH2•CO•OH.
These two compounds are members of one sub-group of the amino acids in which the positive radical is CH3. A second sub-group utilizes the carboxyl radical COOH in the positive position. The simplest compound of this type is d-aspartic acid, COOH•CH2•CHNH2•CO•OH. The third of the sub-groups, the diamino acids, has amine radicals in both the positive and negative positions, as in d-lysine, CH2NH2•(CH2)3•CHNH2•CO•OH.
Another combination containing nitrogen is the cyanide, or nitrile, radical. In the normal radical CN nitrogen has the negative valence three and carbon has the primary magnetic valence two, the net group valence being -1. The positive and negative roles are reversed in the radical NC2 in which nitrogen has the enhanced neutral valence three. In this orientation nitrogen has Division III properties, and is positive to carbon rather than negative as usual. Since the negative valence of carbon is four, the net valence of the radical NC is -l, identical with the valence of CN. The NC compounds, the isocyanides, therefore have the same composition as the cyanides, but different properties.
The CN+ radical makes its appearance in such compounds as cyanoacetic acid, CN•CH2•CO•OH. Here nitrogen is negative, as in the CN• radical, but carbon has the normal positive valence four, and the net group valence is therefore +1. Cyanogen, CN•CN, is a combination of the + 1 and -1 radicals. Compounds with the CO•CN combination in the negative position are nat generally regarded as constituting a separate family, and are named as members of the normal cyanides.
Introduction of the CO neutral group in conjunction with NH2 produces an amide, a structure which is open to an unusually wide variety of additions and substitutions. If we start with acetamide, CH3•CO•NH2, we may add CH2 groups in the normal manner to form propionamide, CH3•CH2•CO•NH2, and the higher homologs, or we may substitute positive radicals for the amine hydrogen, obtaining compounds like N-ethy. acetamide, CH3•CO•(NH•CH2•CH3). The NH combination, which has a net valence of -2, can take the place of oxygen in the CO group of the amide, forming a CNH neutral group which has similar properties. Such a replacement in acetamide gives us acetamidine, CH3•CNH•NH2. If the neutral CO group in acetamide is replaced by the positive CO radical we obtain aminoacetone, COCH3•CH2•NH2. Further replacement of carbon by nitrogen then changes the radical COCH3 to CONH2, and produces a whole new series: urea, CONH2•NH2, and its derivatives. Another CO group changes the monobasic carbamide, urea, to a dibasic compound, oxamide, CONH2•CO•NH2.
A negative combination of oxygen and nitrogen that can be substituted for hydrogen is the nitro group, NO2. This results in a family known as the nitroparaffins. 1-nitropropane, CH3•(CH2)2•NO2, is typical. The NO group in these nitroparaffins is a combination of positive nitrogen (valence +3) with negative oxygen (-2 each). An isomeric family of compounds, the alkyl nitrites, substitutes a group ONO, in which one oxygen atom with the enhanced neutral valence +4 and a nitrogen atom with its normal -3 valence form a valence one positive radical ON. A further combination with negative oxygen then produces a valence one negative radical ONO. The CO•NO2 combination, like CO•CO, is outside the magnetic neutral limits under ordinary conditions, and there is no CO•NO2 series of compounds corresponding to those based on CO•NH2.
In the quaternary ammonium compounds nitrogen has its neutral valence five, as in the inorganic nitrates, and joins with the equivalent of five valence one negative atoms or radicals to form compounds ranging from simple combinations such as tetramethylammonium hydroxide, N(CH3)4•OH, to some very complex, and biologically important, compounds such as lecithin. The quaternary ammonium portion of the lecithin molecule also exists separately as choline, N(CH3)3OH•CH2•CH2•OH.
Addition of oxygen to the cyanide and isocyanide radicals produces the radicals OCN and ONC, which form the basis of the cyanates and isocyanates. A comparison of the cyanides and cyanates provides a good illustration of the way in which the various pertinent factors enter into the construction of chemical compounds. Each element has several possible rotational orientations which it can assume to form chemical combinations, and in each of these orientations it has an effective speed displacement, or valence, which determines the status that the element can assume in a compound, and the ratio in which it combines with the other components. Some orientations are inherently more probable than others, but the type of combination that will be the most stable cannot be determined solely on the basis of this probability, since other factors also enter into the situation. The limitation imposed on direct combinations by the relative negativity of the constituents is one such factor. The greater relative probability of low net group valences in the radicals is another. Replacement capacity is likewise a significant factor. A valence one radical is not only an inherently more probable structure than one of higher valence; it also has an ability to replace hydrogen atoms quite freely, while radicals of higher valence can accomplish such replacements only with some difficulty: In an environment favorable to these replacements the valence one radical therefore takes precedence, if such a radical can be formed.
In any particular instance where there are two or more possible ways of constructing a valence one radical, the combined influence of all effective factors determines which of the possible combinations has the greatest over-all probability, and consequently the greatest stability. Where the margin of one structure over another is small, both may exist under appropriate conditions; where it is large, only the more stable compound can exist. In the cyanides the net total of all factors affecting the combination of carbon and nitrogen favors carbon valence +2 and nitrogen valence -3. An alternate with carbon -4 and nitrogen +3 is close enough to be stable. When oxygen, with valence -2, is added to either of these radicals the positive valence must increase by two units if the addition product is to be a valence one substitute for negative hydrogen. This is possible in both cases, as both carbon and nitrogen have the required higher valences. Carbon steps up from the primary magnetic valence +2 in CN to the normal valence +4 in OCN. Nitrogen goes from the enhanced neutral valence +3 in NC to the neutral valence +5 in ONC. The negative valences are unchanged: nitrogen has -3 in both CN and OCN, carbon has -4 in NC and ONC.
The participation of elements of the higher rotational groups in chemical compounds involves no new structural features. Because of factors such as the higher magnetic valences, the greater inter-atomic distances, and the prevalence of three-dimensional force distributions, in the higher rotational groups, these elements are excluded from many of the types of combinations and structures in which the elements of Group 2A participate. But to the extent to which these elements can occupy positions in such combinations and structures, they do so on the same basis as the analogous Group 2A elements. The descriptions of the various types of combinations and structures in the preceding pages therefore apply to the compounds of these higher group elements as well as to those of the elements that were specifically mentioned.
Sulfur comes the nearest to duplicating the lower group structures. The corresponding Group 2A element, oxygen, uses its negative valence almost exclusively, and to the extent that its somewhat greater inter-atomic distances will permit, sulfur, which has the same -2 valence, duplicates the oxygen compounds. Corresponding to the alcohols, acids, ethers, amides, etc. which have been discussed in the preceding pages, there are thioalcohols, thioacids, thioethers, thioamides, etc., that are identical except that sulfur substitutes for oxygen.
The inter-atomic distance C-S is greater than the C-O distance, and this makes the sulfur compounds somewhat less stable than their oxygen analogs, limiting the total number of these compounds rather severely. One significant point is that the C-S distance will not permit the formation of CS neutral groups, and replacement of neutral CO by CS. This eliminates the possibility of families of sulfur compounds similar to the oxygen families whose negative radicals are CO•OH, CO•NH2, CO•OCH3, and so on. There are thioacids, but the radical is not CS•OH, or CS•SH; it is CO•SH. Where the formula of a compound, as written in accordance with current practice, appears to indicate the presence of a CS group in a neutral position, this is actually a valence two combination that forms part of the positive radical. Thus thioacetamide and thiourea, commonly represented as CH3•CS•NH2 and NH2•CS•NH2, are actually CSCH3•NH2 and CSNH2•NH2. Neither CSOH nor CSSH is barred from acting as a valence one positive radical, a position in which the inter-atomic distance is not a controlling factor, but both are limited in their stability. CSOH tends to rearrange to the more probable form COSH2 while CSSH is vulnerable to loss of a CS molecule. For example, xanthic acid, CSSH•(O•CH2•CH3) spontaneously separates into CS and ethyl alcohol.
Oxidation of the sulfides provides another example of the displacement of the valences by addition of a strongly negative element. In methyl sulfide, (CH3)2S, sulfur has its normal negative valence, -2. Because it is positive to oxygen, oxidation forces it into the positive position in the compound, with a +4 valence, and the CH3 groups, which can take either +1 or -1, shift to the negative. The product is methyl sulfoxide, SO(CH2•H)2. An additional oxygen atom is accommodated by a further shift in the sulfur valence to its maximum value +6 (the neutral valence). The new compound that is formed is methyl sulfone, SO(CH2•H)2.
The single element radicals, such as N3(N+5•N-3•N-3) and C2 (C+2• C•-4) conform to the same pattern of behavior as the other radicals. These particular combinations form azides and carbides respectively. The latter, since they contain no element other than carbon and hydrogen, have been named as a hydrocarbon family, although from a structural standpoint the introduction of the C2 radical into a normal hydrocarbon is the equivalent of the substitution of any other radical, and the resulting compounds should logically be called carbides. The carbide structure is quite evident in such compounds as (CH•CH2)2•C2, which is divinylacetylene, or 1,5 hexadien-3-yne. The valence balance here is the same as in the binary carbides: CaC2, etc. As indicated earlier, however, probability considerations favor valence one radicals, where such radicals are possible, and in the hydrocarbons the C2, combination generally joins with a positive hydrogen atom to form the valence one radical C2H, structurally analogous to OH. The compounds utilizing this radical may be either olefinic (example: vinylacetylene, CH•CH2•C2H) or acetylenic (example: butadiyne, C•CH•C2H). Magnetic neutral groups can be added in the usual manner, forming compounds such as 1,5 hexadiyne, C•CH•CH2•CH2•C2H. This compound, also known as dipropargyl, is isomeric with benzene, and attracted a great deal of attention in the early days of structural chemistry when the “benzene problem” was the center of attention.
A simple carbide, H•C2H, is the initial product of the action of water on calcium carbide, but since hydrogen is negative to carbon a direct combination of this kind between carbon and positive hydrogen is unstable, and the hydrogen carbide promptly changes to acetylene, in which the hydrogen atoms are negative. The valence changes in this series of reactions are interesting. In the original calcium carbide the valences are Ca+2, C+2, C-4. The reaction with water substitutes two +1 hydrogen atoms for the calcium. The relative negativity of carbon and hydrogen then forces hydrogen into the negative position, and since the total negative valence on this basis is only two units, carbon has to take its +1 valence to reach an equilibrium.
Although the three-dimensional inorganic radicals of the SO4 type are not able to substitute freely for hydrogen in organic compounds in the manner of the organic radicals, it is possible for organic chains to replace the atoms that are joined to these three-dimensional radicals in the inorganic compounds. In other words, there is no room for a three-dimensional component in a two-dimensional structure, but a two-dimensional combination can occupy a position in a three-dimensional structure. Typical compounds are ethyl sulfate, (CH3•CH2)2•SO4, and methyl phosphate, (CH3)3•PO4.
Compounds of the metals with organic radicals are usually grouped in a separate category as metal-organic, or organometallic, but they are classified as organic in this work, inasmuch as they have the regular organic structure. A compound such as ethyl sodium, Na•CH2•CH3, has exactly the same structure as the corresponding paraffin hydrocarbon, propane, CH3•CH2•CH3. A compound such as diphenyl tin has exactly the same structure as diphenyl methane, one of the aromatic ring compounds that we will examine in Chapter 21. No separate consideration needs to be given, therefore, to either the organometallic compounds, or those compounds which have both organic and inorganic components, in this discussion of molecular structure.
The number and diversity of the chain compounds can be increased enormously by additional branching, by combinations of the various substituents that have been discussed, and by the use of some less common substituents, but all such compounds follow the same structural principles that have been outlined for the most common organic chain families. There are some additional ways in which structural variations can occur, and to complete the molecular picture a few comments on these items are advisable, but since they are equally applicable to the ring compounds it will be appropriate to defer this discussion until after we have examined the ring structures.
