Chapter 4
Compressibility
One of the simplest physical phenomena is compression, the response of the time region equilibrium to external forces impressed upon it. With the benefit of the information developed earlier, we are now in a position to begin an examination of the compression of solids, disregarding for the present the question of the origin of the external forces. For this purpose we introduce the concept of pressure, which is defined as force per unit area.
| P = F/s2 |
(4-1) |
In many cases it will be convenient to deal with pressure on a volume basis rather than on an area basis. We therefore multiply both force and area by distance, s, which gives us the alternative equation:
| P = Fs/s3 = E/V |
(4-2) |
In the region outside unit distance, where the atoms or molecules of matter are independent, the total energy of an aggregate can thus be expressed in terms of pressure and volume as:
| E = PV |
(4-3) |
As we will find in the next chapter when we begin consideration of thermal motion, a condition of constant temperature is a condition of constant energy, other things being equal. Equation 4-3 thus tells us that for an aggregate in which the cohesive forces between the atoms or molecules are negligible, an ideal gas, the volume at constant temperature is inversely proportional to the pressure. This is Boyle’s law, one of the well-established relations of physics.
For application to the time region in which the solid equilibrium is located, the second power of the volume must be substituted for the first power, in accordance with the general inter-regional relation previously established. The time region equivalent of Boyle’s Law is therefore:
| PV2 = k |
(4-4) |
In terms of volume this becomes
| V = k/P½ |
(4-5) |
This equation tells us that at constant temperature the volume of a solid is inversely proportional to the square root of the pressure. The pressure represented by the symbol P in this equation is, of course, the total effective pressure; that is, the pressure equivalent of all of the forces acting in opposition to the rotational forces of the atom. The force due to the progression of the natural reference system opposes the rotational forces, and acts in parallel with the external compressive forces, but it has the same magnitude regardless of whether or not any such external forces are present. It therefore exerts what we may call an internal pressure, an already existing level of pressure to which an external pressure becomes an addition. In order to conform to established usage and to avoid confusion, the symbol P will hereafter refer to the external pressure only, the total pressure being expressed as P0 + P. On this basis, equation 4-5 may be restated as:
| V = k/(P0+P)½ |
(4-6) |
Compression is normally expressed in terms of relative rather than absolute volumes, the reference volume being the volume at zero external pressure, where equation 4-6 has the form:
| V = k/P0½ |
(4-7) |
Dividing the equation 4-6 by equation 4-7, and rearranging, we obtain:
| V / V0 = P0½ / (P0+P)½ |
(4-8) |
As this equation brings out, the internal pressure, P0, is the key factor in the compression of solids. Inasmuch as this pressure is a result of the progression of the natural reference system which, in the time region, is carrying the atoms inward in opposition to their rotational forces (gravitation), the inward force acts only on two dimensions (an area), and the magnitude of the pressure therefore depends on the orientation of the atom with respect to the line of the progression. As indicated in connection with the derivation of the inter-regional ratio, there are 156.44 possible positions of a displacement unit in the time region, of which a fraction az represents the area subjected to pressure, a and z being the effective displacements in the active dimensions. The letter symbols a, b, and c, are used as indicated in Chapter 10, Volume I. The displacement z is either the electric displacement c or the second magnetic displacement b, depending on the orientation of the atom.
From the principle of equivalence of natural units it follows that each natural unit of pressure exerts one natural unit of force per unit of cross-sectional area per effective rotational unit in the third dimension of the equivalent space. However, the pressure is measured in the units applicable to the effect of external pressure. The forces involved in this pressure are distributed to the three spatial dimensions and to the two directions in each dimension. Only those in one direction of one dimension—one sixth of the total—are effective against the time region structure. Applying this 1/6 factor to the ratio az/156.444, we have for the internal pressure per rotational unit at unit volume,
| P0 = az/938.67 |
(4-9) |
This expression may now be generalized to apply to y rotational units and V units of volume, as follows:
| P0 = azy/(938.67V) |
(4-10) |
The force exerted by the progression of the natural reference system is independent of the geometrical arrangement of the atoms, and the volume term in equation 4-10 refers to what we may call the three-dimensional atomic space, the cube of the inter-atomic distance, rather than to the geometric volume. We will therefore replace V by S03. This gives us the internal pressure equation in final form:
| P0 = azy/(936.67S03) |
(4-11) |
The value derived from this equation is the magnitude of the internal pressure in terms of natural units. To obtain the pressure in terms of any conventional system of units it is only necessary to apply a numerical coefficient equal to the value of the natural unit of pressure in that system. This natural unit was evaluated in Volume I as 5.282 x 1012 dynes/cm2. The corresponding values in the systems of units used in the reports of the experiments with which comparisons will be made in this chapter are:
- 1.554×107 atm
- 1.606×107 kg/cm2
- 1.575×107 megabars
In terms of the units used by P.W. Bridgman, the pioneer investigator in the field, in most of his work, equation 4-11 takes the form
| P0 =17109 azy/S03 kg/cm² |
(4-12) |
The internal pressure thus calculated for any specific substance is not usually constant through the entire external pressure range. At low total pressures, the orientation of the atom with respect to the line of progression of the natural reference system is determined by the thermal forces which, as we will see later, favor the minimum values of the effective cross-sectional area. In the low range of total pressures, therefore, the cross-section is as small as the rotational displacements of the atom will permit. In accordance with Le Chatelier’s Principle, a higher pressure, either internal or external, applied against the equilibrium system causes the orientation to shift, in one or more steps, toward higher displacement values. At extreme pressures the compressive force is exerted against the maximum cross-section: 4 magnetic units in one dimension and 8 electric units in another. Similarly, only one of the magnetic rotational units in the atom participates in the radial component y of the resistance to compression at the low pressures, but further application of pressure extends the participation to additional rotational units, and at extreme pressures all of the rotational units in the atom are involved. The limiting value of y is therefore the total number of such units. The exact sequence in which these two kinds of factors increase in the intermediate pressure range has not yet been determined, but for present purposes a resolution of this issue is not necessary, as the effect of any specific amount of increase is the same in both cases.
Helium and neon, the first two of the inert gases, the elements that have no effective rotation in the electric dimension, take the absolute minimum compression factors: one rotating unit with one effective unit of displacement in each of the two effective dimensions. The azy factors for these elements can be expressed as 1-1-1. In this notation, which we will use for convenience in the subsequent discussion, the numerical values of the compression factors are given in the same order as in the equations. It should be noted that the absolute minimum compression, that applicable to the elements of least displacement, is explicitly defined by the factors 1-1-1. The value of the factor a increases in the higher members of the inert gas series because of their greater magnetic displacement.
Because of their negative displacement in the electric dimension, which, in this context, is equivalent to the zero displacement of the inert gases, the electronegative elements follow the inert gas pattern, taking the minimum 1-1-1 factors in the lowest members of the lowest rotational groups, and values that are higher, but still generally well below those of the corresponding electro-positive elements, as the displacement increases in either or both of the atomic rotations. None of the elements of the electronegative divisions below electric displacement 7 has the 4-8 az factors initially, although they are capable of these high levels, and can eventually reach them under appropriate conditions.
All of the electropositive elements studied by Bridgman have the full 4 units in one dimension; that is, a = 4. The value of z for the alkali metals is equal to the electric displacement, one unit, and since y takes the minimum value under low pressure conditions, the compression factors for these elements are 4-1-1. The displacement 2 elements (calcium, etc.) take the intermediate values 4-2-1 or 4-3-1. The greater displacements of the elements that follow have a double effect. They increase the internal pressure directly by enlarging the effective cross-section, and this higher internal pressure then has the same effect as a greater external pressure in causing a further increase in the compression factors. Most of these elements therefore utilize the full displacements of the active cross-section dimensions from the start of compression; that is, 4-4-1 (az = ab, two magnetic dimensions) in some of the lower group elements and the transition elements of Group 4A, and 4-8-1, or 4-8-n (az = ac, one magnetic and one electric dimension) in the others.
The factors that determine the internal pressures of the compounds that have been investigated thus far fall mainly in the intermediate range, between 4-1-1 and 4-4-1. NaCl, for instance, has 4-2-1 initially, and shifts to 4-3-1 in the pressure range between 30 and 50 M kg/cm2. AgCl has 4-3-1 initially, and carries these factors up to a transition point near Bridgman’s pressure limit of 100 M kg/cm2. CaF2 has the factors 4-4-1 from the start of compression. The initial values of the internal pressure of most of the inorganic compounds examined in this investigation are based on one or another of these three patterns. Those of the organic compounds are mainly 4-1-1, 4-2-1, or an intermediate value 4-1½-1.
Compression is ordinarily measured in terms of relative volume, and most of the discussion in this chapter will deal with the subject on this basis, but for some purposes we will be interested in the compressibility, the rate of change of volume under pressure. This rate is obtained by differentiating equation 4-8.
| (1 / V0) (dV / dP) = P0½ / (2 (P0 + P )3/2) |
(4-13) |
The compressibility at P0, the initial compressibility, is of particular interest. For all practical purposes it is the same as the compressibility at one atmosphere, this pressure being only a small fraction of the internal pressure P0. The initial compressibility may be obtained from equation 4-13 by letting P equal zero. The result is:
| 1 / V0 (dV / dP (P=0)) = 1 / (2P0) |
(4-14) |
Since the initial compressibility is a quantity that can be measured, its simple and direct relation to the internal pressure provides a significant confirmation of the physical reality of that theoretical property of matter. Initial compressibility factors derived theoretically for those elements on which consistent compressibility data are available for comparison, the internal pressures calculated from these factors, and the initial compressibilities corresponding to the calculated internal pressures are listed in Table14, together with measured values of the initial compressibility at room temperature. Two sets of experimental values are given, one from Bridgman and one from a more recent compilation. Values of S03, except those marked with asterisks, are computed from the inter-atomic distances (S0) in the tables of Chapter 2. Where the structure is anisotropic the S03 value shown is the product of one of the distances given in the earlier tabulations by the square of the other. The reason for the occurrence of the indicated deviations from the Chapter 2 values will be explained later.
Table 14: Initial Compressibility
In most cases the difference between the calculated and measured compressibilities is within the probable experimental error. Substantial deviations from the calculated values are to be expected in the case of low melting point elements such as the alkali metals, unless corrections have been applied to the empirical data, as there is an additional component in the initial volume of such substances. Elsewhere, the differences between the calculated compressibilities and either of the two sets of experimental values are, on the average, no greater than the differences between the experimental results. This process is repeated at successively higher pressure levels until the maximum compression factors for the element are reached.
Because of the nature of this compression pattern, a convenient method of analyzing the experimental values of the volume of various substances under compression can be made available by expressing equation 4-8 in the form:
| (V0 /V)2 = 1+P/P0 |
(4-15) |
According to this equation, if we plot the reciprocals of the squares of the relative volumes against the corresponding total pressure ratios we should obtain a straight line intersecting the zero pressure ordinate at the reference volume 1.00. The slope of the line is determined by the magnitude of the internal pressure, P0 Figure 1(a) is a curve of this kind for the element tin, based on Bridgman’s experimental values.
Figure 1: Compression Patterns
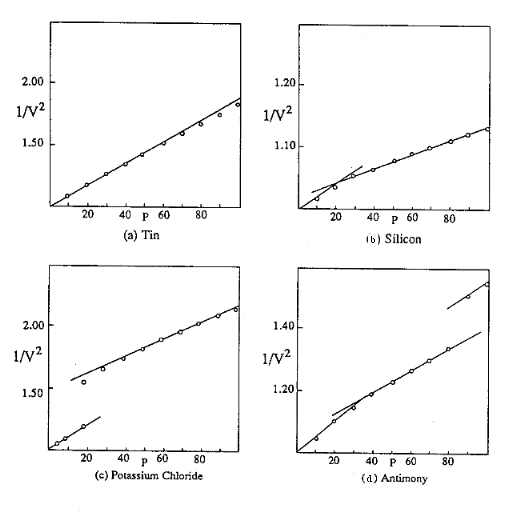
Where there is a transition to a higher set of compression factors within the experimental range, and the magnitude of P0 changes, the volumes diverge from the original line and follow a second straight line, the slope of which is determined by the new compression factors. On preparing curves of this kind for the other elements investigated by Bridgman, we find that about two-thirds of them actually do conform to a single straight line up to the 30,000 kg/cm2 pressure limit of his earlier work. His studies of the less compressible substances, such as the higher elements of the electropositive divisions, were not carried beyond this level, but he measured the compression up to 100,000 kg/cm2 on many other elements, and most of them were found to undergo a transition in which the effective internal pressure increases without any volume discontinuity. The compression curve for such a substance consists of two straight line segments connected by a smooth transition curve, as in Figure 1(b), which represents Bridgman’s values for silicon.
In addition to the changes of this type, commonly called second order transitions, some solid substances undergo first order transitions in which there is a modification of the crystal structure and a volume discontinuity at the transition point. The effective internal pressure generally changes during a transition of this kind, and the resulting volumetric pattern is similar to that of KCl, Figure 1(c). With the exception of some values which are rather erratic and of questionable validity, all of Bridgman’s results follow one of these three patterns or some combination of them. The antimony curve, Figure 1(d), illustrates one of the combination patterns. Here a second order transition between 30,000 and 40,000 kg/cm2 is followed by a first order transition at a higher pressure. The numerical values corresponding to these curves are given in the tables that follow.
The experimental second order curves are smooth and regular, indicating that the transition process takes place freely when the appropriate pressure is reached. The first order transitions, on the other hand, show considerable irregularity, and the experimental results suggest that in many substances the structural changes at the transition points are subject to a variable amount of delay due to internal conditions in the solid aggregate. In these substances the transition does not take place at a well-defined pressure, but somewhere within a relatively broad transition zone, and the exact course of the transition may vary considerably between one series of measurements and another. Furthermore, there are many substances which appear to experience similar delays in achieving volumetric equilibrium even where no transitions take place. The compression curves suggest that a number of the reported transitions are actually volume adjustments which merely reflect delayed response to the pressure applied earlier. For example, in the barium curve based on Bridgman’s results there are presumably two transitions, one between 20,000 and 25,000 kg/cm2, and the other between 60,000 and 70,000 kg/cm2. Yet the experimental volumes at 60,000 and 100,000 kg/cm2 are both very close to the values calculated on the basis of a single straight line relation. It is quite probable, therefore, that this element actually follows one linear relation at least up to the vicinity of 100,000 kg/cm2.
The deviations from the theoretical curves that are found in the experimental volumes of substances with relatively high melting points are generally within the experimental error range, and those larger deviations that do make their appearance can, in most cases, be explained on the foregoing basis. The compression curves for substances with low melting points show systematic deviations from linearity at the lower pressures, but this is a normal pattern of behavior resulting from the proximity of the change of state. As will be brought out in detail in our examination of the liquid state, the physical state of matter is basically a property of the individual atom or molecule. The state of the aggregate merely reflects the state of the majority of its individual constituents. Consequently, a solid aggregate at any temperature near the melting point contains a specific proportion of liquid molecules. Since the volume of a liquid molecule differs from that of a solid molecule, the volume of the aggregate is modified accordingly. The amount of the volume deviation in each case can be calculated by methods that will be described in the subsequent discussion of the liquid volume relations.
Table 15 compares the results of the application of equation 4-8 with Bridgman’s measurements on some of the elements that maintain the same internal pressure all the way up to his pressure limit of 100,000 kg/cm2. In many cases he made several series of measurements on the same element. Most of these results agree within about 0.003, and it does not appear that listing all of the individual values in the tabulations would serve any useful purpose. The values given in Table15, and in the similar tables that follow, are those obtained in experiments that were carried to the full 100,000 kg/cm2 pressure level. Where the high pressure measurements were started at some elevated pressure, or where the measurement interval was greater than usual, the gaps have been filled from the results of other Bridgman experiments.
Table 15: Relative Volumes Under Compression
| Pressure (M kg/cm2) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Zn 4-4-1 |
Zr 4-6-1½ |
In 4-4-1 |
Sn 4-4-1 |
|||||
| Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | |
| 0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 5 | 0.992 | 0.992 | 0.995 | 0.995 | 0.988 | 0.988 | 0.992 | 0.991 |
| 10 | 0.984 | 0.984 | 0.990 | 0.989 | 0.980 | 0.980 | 0.984 | 0.982 |
| 15 | 0.976 | 0.977 | 0.985 | 0.983 | 0.970 | 0.967 | 0.976 | 0.975 |
| 20 | 0.969 | 0.969 | 0.980 | 0.978 | 0.960 | 0.955 | 0.968 | 0.966 |
| 25 | 0.961 | 0.964 | 0.975 | 0.973 | 0.951 | 0.948 | 0.961 | 0.960 |
| 30 | 0.954 | 0.954 | 0.970 | 0.969 | 0.942 | 0.936 | 0.954 | 0.951 |
| 35 | 0.947 | 0.965 | 0.964 | 0.933 | 0.932 | 0.947 | ||
| 40 | 0.940 | 0.939 | 0.960 | 0.960 | 0.925 | 0.919 | 0.940 | 0.936 |
| 50 | 0.927 | 0.925 | 0.951 | 0.946 | 0.909 | 0.903 | 0.926 | 0.923 |
| 60 | 0.914 | 0.912 | 0.942 | 0.937 | 0.893 | 0.888 | 0.913 | 0.909 |
| 70 | 0.902 | 0.900 | 0.933 | 0.929 | 0.878 | 0.874 | 0.901 | 0.897 |
| 80 | 0.890 | 0.889 | 0.925 | 0.922 | 0.864 | 0.860 | 0.889 | 0.886 |
| 90 | 0.879 | 0.878 | 0.917 | 0.916 | 0.851 | 0.847 | 0.878 | 0.875 |
| 100 | 0.868 | 0.868 | 0.909 | 0.910 | 0.838 | 0.835 | 0.867 | 0.864 |
Table16 extends the volume comparisons to representative elements of the classes that are subject to transitions within the experimental range of pressures. Transitions reported by the investigator or indicated by the theoretical calculations are shown by horizontal lines in the appropriate columns. In these tabulations the position of the upper branch of each curve has been fixed by using the experimental volume at a selected pressure in the straight line segment above the transition (identified by the symbol R) as a reference point. Thus the slope of this upper branch of the curve is determined theoretically, but its position relative to the 1/V2 scale is empirical. Some work has been done toward extension of the theoretical development to a determination of the exact position of the upper section of each curve, but this project is not far enough advanced to justify any discussion of it at this time.
Table 16: Relative Volumes Under Compression
| Pressure (M kg/cm2) |
Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. |
|---|---|---|---|---|---|---|---|---|
| Al 4-5-1 4-8-1 |
Si 4-4-1 4-8-1 |
Ca 4-3-1 4-4-1 |
Sb 4-4-1 4-4-1½ |
|||||
| 0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 5 | .993 | .993 | .996 | .995 | .970 | .969 | .988 | .987 |
| 10 | .987 | .987 | .991 | .990 | .943 | .942 | .977 | .975 |
| 15 | .981 | .981 | .987 | .986 | .917 | .918 | .966 | .964 |
| 20 | .974 | .975 | .982 | .981 | .895 | .897 | .955 | .954 |
| 25 | .968 | .969 | .978 | .978 | .878 | .878 | .945 | .944 |
| 30 | .964 | .964 | .974 | .974 | .862 | .861 | .935 | .934 |
| 35 | .847 | .845 | .925 | .925 | ||||
| 40 | .957 | .958 | .966 | .968 | .832 | .832 | .916 | .917 |
| 50 | .949 | .951 | .960 | .962 | .805 R | .805 | .899 | .899 |
| 60 | .942 | .944 | .956 | .957 | .780 | .780 | .888 | .886 |
| 70 | .935 | .937 | .952 | .952 | .758 | .748 | .875 | .875 |
| 80 | .928 | .929 | .948 | .948 | .737 | .732 | .864 R | .864 |
| 90 | .922 | .922 | .944 | .944 | .718 | .716 | .815 | |
| 100 | .915 R | .915 | .940 R | .940 | .701 | .702 | .803 | |
| Ba 4-2-1 4-3-1 |
La 4-4-1 4-8-1 |
Pr 4-4-1 4-4-1½ |
U 4-8-1 4-8-2 |
|||||
| 0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 5 | .955 | .955 | .982 | .981 | .984 | .983 | .996 | .955 |
| 10 | .915 | .914 | .965 | .963 | .970 | .967 | .991 | .990 |
| 15 | .880 | .879 | .949 | .947 | .955 | .953 | .987 | .986 |
| 20 | .848 | .841 | .933 | .933 | .942 | .940 | .983 | .981 |
| 25 | .820 | .814 | .918 | .917 | .929 | .927 | .979 | .978 |
| 30 | .794 | .789 | .904 | .905 | .916 | .915 | .975 | .973 |
| 35 | .771 | .770 | .891 | .893 | .904 | .904 | .971 | .971 |
| 40 | .750 | .747 | .878 | .881 | .893 | .893 | .967 | .966 |
| 50 | .712 | .712 | .858 | .863 | .878 | .878 | .960 | .960 |
| 60 | .679 | .682 | .845 | .846 | .863 | .863 | .956 | .955 |
| 70 | .650 | .639 | .833 | .832 | .849 R | .849 | .952 | .951 |
| 80 | .625 | .618 | .821 | .819 | .835 | .836 | .949 | .947 |
| 90 | .603 | .598 | .809 | .808 | .822 | .823 | .945 | .944 |
| 100 | .582 | .580 | .798 R | .798 | .810 | .811 | .941 R | .941 |
Compressibility patterns of compounds are theoretically identical with those of the elements, and this theoretical conclusion is confirmed by compression data for a representative group of inorganic compounds presented in Table17.
Table 17: Relative Volumes Under Compression
| Pressure (M kg/cm2) |
Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. |
|---|---|---|---|---|---|---|---|---|
| NaCl 4-2-1 4-2-1½ |
NaI 4-2-1 4-2-1½ |
KCl 4-2-1 4-2-1½ |
ZnS 4-4-1 4-4-1½ |
|||||
| 0 | .994 | 1.000 | .987 | 1.000 | .994 | 1.000 | .995 | 1.000 |
| 5 | .979 | .982 | .964 | .970 | .973 | .974 | .991 | .994 |
| 10 | .964 | .966 | .942 | .944 | .953 | .952 | .986 | .988 |
| 15 | .950 | .951 | .922 | .922 | .934 | .933 | .982 | .982 |
| 20 | .937 R | .937 | .903 | .902 | .916 R | .916 | .977 R | .977 |
| .803 R | .803 | |||||||
| 25 | .924 | .924 | .885 | .886 | .791 | .789 | .973 | .972 |
| 30 | .912 | .912 | .868 | .871 | .779 | .778 | .969 | .967 |
| 35 | .900 | .901 | .853 | .858 | .768 | .768 | .964 | .963 |
| 40 | .889 | .892 | .840 | .840 | .757 | .758 | .960 | .961 |
| 50 | .867 | .865 | .819 | .816 | .741 | .742 | .952 | .954 |
| 60 | .847 | .848 | .799 | .795 | .727 | .723 | .945 | .947 |
| 70 | .829 | .832 | .781 | .777 | .714 | .710 | .940 | .940 |
| 80 | .815 | .817 | .765 | .761 | .702 | .698 | .934 | .934 |
| 90 | .802 | .803 | .749 | .747 | .690 | .688 | .929 | .929 |
| 100 | .790 R | .790 | .734 R | .734 |
.679 R |
.679 | .924 R | .924 |
| AgCl 4-3-1 |
CsBr 4-3-1 4-4-1 |
NH4Cl 4-2-1 4-4-1 |
KNO3 4-3-1 4-3-2 |
|||||
| 0 | 1.000 | 1.000 | .984 | 1.000 | 1.000 | 1.000 | .894 | 1.000 |
| 5 | .990 | .989 | .962 | .971 | .974 | .973 | .878 | .882 |
| 10 | .980 | .979 | .942 | .947 | .950 | .951 | .862 | .862 |
| 15 | .971 | .969 | .923 | .925 | .928 | .933 | .847 | .846 |
| 20 | .961 | .960 | .905 R | .905 | .910 | .918 | .833 | .831 |
| 25 | .952 | .952 | .888 | .888 | .900 | .905 | .820 R | .820 |
| 30 | .944 | .942 | .871 | .870 | .889 | .891 | .807 | .804 |
| 35 | .935 | .937 | .856 | .859 | .879 | .883 | ||
| 40 | .927 | .926 | .842 | .840 | .869 | .867 | .783 | .781 |
| 50 | .911 | .910 | .815 | .814 | .851 | .846 | .761 | .762 |
| 60 | .895 | .896 | .790 | .792 | .833 | .828 | .744 | .745 |
| 70 | .881 | .883 | .777 | .773 | .817 | .812 | .733 | .732 |
| 80 | .867 | .871 | .760 | .757 | .801 | .798 | .723 | .720 |
| 90 | .854 | .860 | .743 | .742 | .787 | .785 | .712 | .711 |
| 100 | .841 | .835 | .728 R | .728 | .773 R | .773 | .703 R | .703 |
As might be expected for the less uniform composition, transitions are somewhat more common in the compounds, but otherwise there is no difference in the compression curves. The curve for KCl, shown graphically in Figure 1 and by numerical values in Table 17, is of special interest because it includes a sharp first order transition in which there is a substantial decrease in the basic volume while the compression factors remain unchanged. The magnitude of the volume reduction that takes place indicates that there is a reorientation of the atomic rotations in which the neutral specific electric rotation 5 is substituted for the normal rotation 4 as the effective relative value. The theoretical volumes beyond the transition point, as shown in the table, are based on the small atomic volume corresponding to the higher rotation. Up to 20,000 kg/cm2 the volume follows the curve corresponding to compression factors 4-2-1 and S03 = 1.222, which produce an internal pressure of 112.7 M kg/cm2. At the transition point the basic volume (S03) drops to 0.976, increasing the internal pressure to 141.1 M kg/cm2. The compression then continues on this basis up to the vicinity of 45,000 kg/cm2, where the compression factors change from 4-2-1 to 4-3-1, and the internal pressure rises accordingly.
As in the compression of the elements, the theoretical calculations do not always confirm the transitions reported by the experimenters. On the other hand, these calculations show that a large proportion of the compounds, including six of the eight in Table 17, undergo either a transition or some other process in which they eliminate a volume component in the pressure range below 5000 kg/cm2. The effect on the compression curve is to cause the linear segment of the curve to intersect the zero pressure ordinate at a volume below 1.000. The origin of these volume adjustments is still uncertain. The occurrence of a number of observable first order transitions at relatively low pressures suggests that some early second order transitions may also be taking place. But it is also possible that voids in the structure may be eliminated in the early stages of compression, or that there are geometrical readjustments.
The structural characteristics of the organic compounds make them particularly susceptible to such geometrical readjustments. Because of their low melting points, their volumes under low pressure also include the additional component that exists near the change of state. It appears, however, that in a wide range of compounds elimination of these extra volume components is substantially complete at some pressure well below the 40,000 kg/cm2 level to which Bridgman’s measurements on solid organic compounds were carried. This means that there is a fairly wide pressure range in which these compounds follow the normal compression pattern. The following comparison of theoretical and observed volume ratios for benzene and some of its polynuclear derivatives gives an indication of how the elimination of the excess volume progresses. A measured ratio lower than the theoretical means that some of the excess volume is eliminated in the pressure range for which the ratio is measured, and the amount of the difference is an indication of the amount by which the normal loss of volume due to compression is increased.
| Benzene | Ratio 40/25 | ||||
|---|---|---|---|---|---|
| P (M kg/cm2) |
Ratio | ||||
| Calc. | Obs. | Calc. | Obs. | ||
| 40/20 | .938 | .920 | Benzene | .954 | .943 |
| 40/25 | .954 | .943 | Naphthalene | .954 | .950 |
| 40/30 | .970 | .964 | Anthracene | .954 | .953 |
| 40/35 | .985 | .984 | |||
As these figures indicate, benzene is just getting rid of the last of the excess volume at the pressure limit of the experiments, and there is no linear section of the benzene compression curve on which the slope can be measured for comparison with the theoretical value. With increased molecular complexity, however, the linear section of the curve lengthens, and for compounds with characteristics similar to those of anthracene there is a 15,000 kg/cm2 interval in which the measured volumes should follow the theoretical line.
Compounds of this nature have magnetic rotation 3-3 and electric rotation 4. The effective value of S03 is therefore 0.812, and where the compression factors are 4-1½-1 the resulting internal pressure is 127.2 M kg/cm2. As shown in the values tabulated for benzene, which were computed on the basis of this internal pressure, the ratio of the volume at 40,000 kg/cm2 to that at 25,000 kg/cm2 should be 0.954 for all organic compounds with characteristics (molecular complexity, melting point, compression factors, etc.) similar to those of anthracene. Table 18 shows that this theoretical conclusion is corroborated by Bridgman’s measurements.
Table 18: Measured Volume Ratio - 40/25 M/kg/cm2
(Theoretical ratio: 0.954)
| Urea | 0.954 | p-Nitroiodobenzene | 0.955 |
| Nitrourea | 0.956 | o-Chlorobenzoic acid | 0.954 |
| Cyanamide | 0.953 | m-Chlorobenzoic acid | 0.953 |
| o-Xylene | 0.956 | p-Chlorobenzoic acid | 0.954 |
| p-Xylene | 0.956 | o-Bromobenzoic acid | 0.954 |
| Triphenyl methane | 0.953 | m-Bromobenzoic acid | 0.954 |
| o-Diphenyl benzene | 0.954 | p-Bromobenzoic acid | 0.954 |
| m-Diphenyl benzene | 0.955 | m-Iodobenzoic acid | 0.955 |
| p-Diphenyl benzene | 0.955 | p-Iodobenzoic acid | 0.953 |
| Chlorobenzene | 0.954 | p-Nitroaniline | 0.954 |
| o-Nitrochlorobenzene | 0.956 | o-Acetyl tuluidine | 0.954 |
| o-Nitrobromobenzene | 0.955 | Tetrahydronaphthalene | 0.953 |
| p-Nitrobromobenzene | 0.953 | Anthracene | 0.953 |
| o-Nitroiodobenzene | 0.953 | Acenaphthene | 0.955 |
At the time the theoretical values listed in the foregoing tables were originally calculated, Bridgman’s results constituted almost the whole of the experimental data then available in the high pressure range, and his experimental limit at 100,000 kg/cm2 was the boundary of the empirical knowledge of the effect of high pressure. In the meantime the development of shock wave techniques by American and Russian investigators has enabled measuring compressions at pressures up to several million atmospheres. With the benefit of these new measurements we are now able to extend the correlation between theory and experiment into the region of the maximum compression factors.
The nature of the response of the compression factors to the application of pressure has already been explained, and the maximum factors for each group of elements have been identified. However, the magnitude of the base volume (S03) also enters into the determination of the internal pressure, and coincidentally with the increase in these factors there is a trend toward a minimum base volume. In themselves, modifications of the crystal structure play only a small part in the compressibility picture. Application of sufficient pressure causes a solid to assume one of the crystal forms corresponding to the closest packing of the atoms, the face-centered cubic or close-packed hexagonal for isometric crystals, and the nearest equivalent structures if the crystals are anisometric. If some different crystal form exists at zero pressure, the volume decrease due to the change to one of the close-packed forms shows up as a percentage reduction in all subsequent volumes, but the compressibility is not otherwise affected. However, a difference in crystal structure often indicates a difference in the relative orientation of the atomic rotations. Any such change in orientation alters the internal pressure, and consequently has a significant effect on the compressibility.
Application of pressure tends to favor what may be called “regular” structures at the expense of those structures that are able to exist only because of special conditions applicable to the particular elements involved. This tendency is evident from the start of the compression process, and is responsible for the large number of deviations from the Chapter 2 values of the inter-atomic distances that are identified by asterisks in Table 14. For example the five elements from chromium to nickel have a number of different inter-atomic distances at low pressure, and are able to crystallize in alternate forms. In the early stages of compression, however, all of these elements, except manganese, orient themselves on the basis of the neutral relative rotation 10, and have an internal pressure that reflects the corresponding value of S03, which is 0.603. At still higher pressures vanadium shifts to the same relative rotation and joins the group. Manganese probably does likewise, but empirical confirmation of this change is still lacking. Thus the change of variation of the atomic arrangements is greatly reduced by external pressure. One of the collateral effects is that the amount of uncertainty in the identification of the rotation orientation, and the resulting base volume, is minimized.
Most of the elements that change to a lower base volume at the start of compression maintain this new value of S03 throughout the remainder of the present range of the shock wave experiments. Those that do not make this change in the early stages of compression generally do so at some higher pressure. Only a few keep the same base volume up to the shock wave pressure limit. Still fewer undergo a second transition to a lower base volume. Thus the general pattern involves one reduction of the base volume in the pressure range from zero external pressure up to the limit of the shock wave experiments. This pattern is reflected in the twelve series of measurements that have been selected for comparison with the theoretical values. Out of the twelve elements that are included, only two, copper and chromium, have the same base volume in the shock wave range as at zero pressure. Four continue with the values of S03 applicable to the early stages of compression, the values listed in Table 14, and six change to a lower base volume somewhere above Bridgman’s pressure limit. The minimum base volumes, the corresponding maximum compression factors, and the resulting internal pressures for these elements are shown in Table 19.
Table 19: Maximum Internal Pressures
| c | a-b | S03 | a-z-y | P0 | c | a-b | S03 | a-z-y | P0 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V | 10 | 4-3 | 0.603 | 4-8-2 | 1816 | Ag | 8-10 | 4-4 | 0.823 | 4-8-4 | 2661 |
| Cr | 10 | 4-3 | 0.603 | 4-8-3 | 2724 | W | 10 | 4-4½ | 0.822 | 4-8-5 | 3330 |
| Co | 10 | 4-3 | 0.603 | 4-8-3 | 2724 | Au | 10 | 4-4½ | 0.822 | 4-8-5 | 3330 |
| Ni | 10 | 4-3 | 0.603 | 4-8-3 | 2724 | Tl | 5-10 | 4-4½ | 1.074 | 4-8-5 | 2549 |
| Cu | 8-10 | 4-3 | 0.652 | 4-8-3 | 2519 | Pb | 5-10 | 4-4½ | 1.074 | 4-8-5 | 2549 |
| Mo | 10 | 4-4 | 0.764 | 4-8-4 | 2866 | Th | 5 | 4½-4½ | 1.631 | 4-8-5 | 1678 |
Here again, as in the pressure range of the Bridgman experiments, the theoretical development is not yet far enough advanced to enable specifying the exact locations of the upper sections of the compression curves. Nor is it yet clear in all cases just how many of the possible intermediate values of the compression factors are actually utilized as the pressure increases. What we are able to do at the present rather early stage of the development of the theory is to demonstrate that in this extreme high pressure range, as well as at the lower pressures of the preceding tables, the volume varies inversely with the square root of the total pressure, strictly in accordance with the theory. In this connection it should be noted that the section of each compression curve that is based on the maximum value of the internal pressure is long enough to make the square root pattern clear and distinct.
Furthermore, we are able to show that the slope of the last section of the experimental curve for each element is identical with the theoretical slope determined by the calculated maximum values of the internal pressure, and that the slope of each of the intermediate sections is in agreement with one of the possible intermediate values of that internal pressure. An exact theoretical definition of the curves will have to wait for further progress along the lines discussed earlier. In the meantime, the amount of theoretical information already available will serve as a means of testing the validity of each set of empirical results, and will also enable a reasonable amount of extrapolation of the compression curves beyond the present limits of the shock wave technology.
Table 20 is a comparison of the theoretical volumes, based on an empirical reference volume for each of the sections of the curves, as in the preceding tables, with the shock wave results obtained at Los Alamos5 on the elements that were investigated over the widest range of pressures. Unless there is an increase in the compression factors in the vicinity of 100,000 atmospheres, the compression curves established on the basis or Bridgman’s measurements extend into the lower range of these shock wave experiments. In these cases the theoretical volumes up to the first change in the compression factors are calculated on the basis of the reference volume selected from the Bridgman data, and no reference point is identified in this table.
Table 20: Shock Wave Compressions
| P | a-z-y | Calc. | Obs. | a-z-y | Calc. | Obs | a-z-y | Calc. | Obs. |
|---|---|---|---|---|---|---|---|---|---|
| W | Au | Mo | |||||||
| 0.1 | 4-8-3 | .972 | .970 | 4-8-1½ | .946 | .953 | 4-8-2 | .966 | .966 |
| .2 | .946 | .944 | 4-8-3 | .911 | .917 | .936 | .937 | ||
| .3 | .922 | .921 | .888 R | .888 | .908 | .912 | |||
| .4 | .900 | .901 | .867 | .864 | 4-8-3 | .885 | .890 | ||
| .5 | 4-8-4 | .880 | .882 | .847 | .843 | .868 | .870 | ||
| .6 | .865 | .866 | .828 | .825 | .851 | .852 | |||
| .7 | .850 | .851 | .811 | .810 | .836 | .836 | |||
| .8 | .836 R | .836 | .794 | .796 | .822 | .821 | |||
| .9 | .823 | .824 | 4-8-5 | .780 | .783 | .808 | .807 | ||
| 1.0 | .810 | .812 | .771 | .772 | .795 R | .795 | |||
| 1.1 | .798 | .800 | .762 R | .762 | .783 | .783 | |||
| 1.2 | 4-8-5 | .787 | .790 | .754 | .752 | .771 | .772 | ||
| 1.3 | .778 | .780 | .745 | .743 | 4-8-4 | .761 | .762 | ||
| 1.4 | .770 | .771 | .737 | .735 | .752 | .752 | |||
| 1.5 | .762 R | .762 | .730 | .728 | .743 R | .743 | |||
| 1.6 | .754 | .754 | .722 | .720 | .734 | .734 | |||
| 1.7 | .747 | .746 | .715 | .714 | .726 | .726 | |||
| 1.8 | .739 | .738 | .708 | .708 | |||||
| 1.9 | .732 | .731 | .701 | .702 | |||||
| 2.0 | .725 | .725 | .694 | .696 | |||||
| 2.1 | .718 | .718 | |||||||
| Cr | Pb | V | |||||||
| 0.1 | 4-8-1½ | .955 R | .955 | 4-4-1½ | .858 | .865 | 4-8-1 | .939 | .945 |
| .2 | .924 | .920 | 4-4-3 | .796 R | .796 | 4-8-1½ | .900 | .902 | |
| .3 | .895 | .891 | .753 | .751 | .867 R | .867 | |||
| .4 | .869 | .867 | .716 | .718 | .838 | .838 | |||
| .5 | .845 | .846 | 4-8-3 | .691 | .693 | .811 | .812 | ||
| .6 | .823 | .827 | .673 R | .673 | .787 | .790 | |||
| .7 | 4-8-3 | .805 | .811 | .656 | .656 | .765 | .770 | ||
| .8 | .794 | .797 | .640 | .642 | 4-8-2 | .750 | .753 | ||
| .9 | .783 | .784 | 4-8-5 | .628 | .630 | .736 | .737 | ||
| 1.0 | .772 R | .772 | .619 R | .619 | .723 R | .723 | |||
| 1.1 | .762 | .761 | .610 | .609 | .710 | .709 | |||
| 1.2 | .752 | .751 | .602 | .600 | .698 | .697 | |||
| 1.3 | .742 | .742 | .594 | .593 | .687 | .686 | |||
| 1.4 | .733 | .733 | .586 | .586 | |||||
| Co | Ni | Cu | |||||||
| 0.1 | 4-8-1½ | .953 | .956 | 4-8-1½ | .953 | .954 | 4-8-1 | .945 | .940 |
| .2 | .921 | .920 | .921 | .919 | .898 | .897 | |||
| .3 | .893 | .890 | .893 | .889 | 4-8-1½ | .865 | .864 | ||
| .4 | .867 | .865 | .867 | .865 | .838 | .836 | |||
| .5 | .843 R | .843 | .843 R | .843 | .814 R | .814 | |||
| .6 | .821 | .823 | .821 | .825 | .792 | .794 | |||
| .7 | .801 | .806 | .801 | .808 | 4-8-3 | .772 | .777 | ||
| .8 | .782 | .791 | 4-8-3 | .790 | .794 | .760 | .762 | ||
| .9 | 4-8-3 | .769 | .776 | .779 | .780 | .749 | .749 | ||
| 1.0 | .759 | .764 | .768 | .768 | .738 | .737 | |||
| 1.1 | .749 | .752 | .758 | .757 | .728 | .726 | |||
| 1.2 | .739 | .741 | .748 | .747 | .718 | .716 | |||
| 1.3 | .730 | .731 | .739 | .738 | .708 | .707 | |||
| 1.4 | .721 | .721 | .730 | .729 | .699 | .698 | |||
| 1.5 | .712 R | .712 | .721 R | .721 | .690 R | .690 | |||
| 1.6 | .704 | .704 | |||||||
| Ag | Tl | Th | |||||||
| 0.1 | 4-8-1 | .922 | .929 | 4-4-3 | .850 | .853 | 4-8-1 | .869 | .870 |
| .2 | 4-8-2 | .879 | .881 | .787 | .783 | 4-8-2 | .792 | .795 | |
| .3 | .848 | .845 | .736 R | .736 | .747 | .744 | |||
| .4 | .820 | .817 | 4-8-3 | .702 | .703 | .710 | .707 | ||
| .5 | .794 R | .794 | .678 R | .678 | .677 R | .677 | |||
| .6 | .771 | .775 | .656 | .658 | 4-8-3 | .652 | .652 | ||
| .7 | 4-8-4 | .752 | .759 | .637 | .642 | .632 R | .632 | ||
| .8 | .741 | .744 | 4-8-5 | .623 | .628 | .613 | .614 | ||
| .9 | .730 | .731 | .614 | .616 | .596 | .599 | |||
| 1.0 | .720 R | .720 | .605 R | .605 | 4-8-5 | .583 | .585 | ||
| 1.1 | .710 | .710 | .597 | .596 | .572 | .573 | |||
| 1.2 | .701 | .700 | .588 | .587 | .562 | .562 | |||
| 1.3 | .692 | .692 | .581 | .580 | .553 | .553 | |||
| 1.4 | .683 | .684 | .573 | .573 | .544 | .544 | |||
| 1.5 | .675 | .677 | .566 | .567 | .535 | .535 | |||
| 1.6 | .667 | .670 | |||||||
A rather surprising feature of these comparisons is that the agreement between the shock wave results and the theoretical volumes is as close as the agreement between Bridgman’s static values and the theory. It is true that this set of measurements was deliberately selected for the comparison, and it represents the best results rather than the average, but in any event the close correlation is a significant confirmation of the validity of both the shock wave techniques and the theoretical relations.
The question that now arises is what course the compressibility follows beyond the pressure range of this table. In some cases a transition to a smaller base volume appears to be possible. Copper, for instance, may shift to the rotations of the preceding electropositive elements at some pressure above that of the tabulation. Aside from such special cases, the factors that determine the compressibility in the range below two million atmospheres have reached their limits. At the present stage of the investigation, however, the possibility that some new factor may enter into the picture at extreme pressures cannot be excluded. A “collapse” of the atomic structure of the kind envisioned by the nuclear theory is. of course, impossible, but as matters now stand we are not in a position to say that all aspects of the compressibility situation have been explored. It is conceivable that there may be some, as yet unknown, capability of change in the atomic motions that would increase the resistance to pressure beyond what now appears to be the ultimate limit.
Some shock wave measurements have been made at still higher pressure levels, and these should throw some light on the question. Unfortunately, however, the results are rather ambiguous. Three of the elements included in these experiments, lead, tin, and bismuth, follow the straight line established in Table 20 up to the maximum pressures of about four million atmospheres. On the other hand, five elements on which measurements were carried to maximums between three and five million atmospheres show substantially lower compressions than a projection of the Table 20 curves would indicate. The divergence in the case of gold, for example, is almost eight percent. But there are equally great differences between the results of different experiments, notably in the case of iron. Whether or not some new factor enters into the compression situation at pressures above those of Table 20 will therefore have to be regarded as an open question.
